Electrochemical cells, the cornerstone of modern energy conversion and storage technologies, embody a fascinating realm where chemistry meets electricity to power our world. From the batteries that fuel our everyday devices to the innovative systems driving green energy solutions, understanding the diversity within these cells reveals the intricate balance of science and technology. Among the various types, concentration cells and chemical cells stand out due to their unique mechanisms and applications, each playing pivotal roles in the advancement of electrochemical research and applications.
A concentration cell generates electrical energy through the differential in concentration of the electrolyte in two half-cells, while a chemical cell, often referred to as a galvanic cell, produces electricity through chemical reactions between different substances. This distinction is fundamental to grasping how electrochemical cells are designed and utilized across various industries, from portable electronics to renewable energy systems.
Diving deeper, concentration cells leverage the gradient in concentration to induce movement of ions and electrons, creating a flow of electrical current without the need for external chemical reactions. On the other hand, chemical cells rely on the spontaneous redox reactions between different electrodes and electrolytes to produce electricity. This intricate dance of electrons not only powers our daily lives but also pushes the boundaries of what is possible in energy storage and conversion technologies.
Electrochemical Cells
Basics
Definition and Function of Electrochemical Cells
Electrochemical cells are devices that convert chemical energy into electrical energy or use electrical energy to cause a chemical reaction. This conversion is fundamental to the operation of batteries, fuel cells, and various forms of sensors. The heart of these cells lies in the redox reactions – where oxidation (loss of electrons) and reduction (gain of electrons) processes occur, facilitating the flow of electrons through an external circuit.
Types
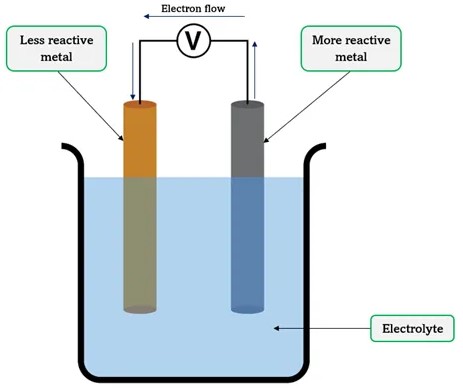
Electrochemical cells come in many forms, each with specific applications and principles of operation. Broadly, they can be classified into galvanic (voltaic) cells, which generate electricity through spontaneous chemical reactions, and electrolytic cells, which use electrical energy to drive non-spontaneous chemical reactions. This discussion primarily focuses on concentration cells and chemical cells, two subsets of galvanic cells, for their distinctive features and widespread applications.
Concentration Cells
Definition
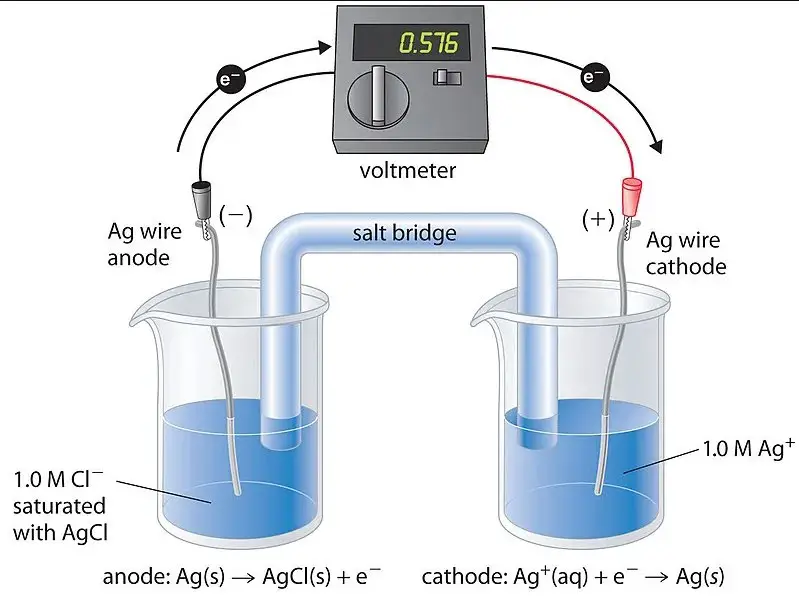
A concentration cell is a type of electrochemical cell where the electrodes are the same material, but they immerse in electrolyte solutions of different concentrations. The main driving force behind these cells is the concentration gradient between the two solutions, leading to the generation of an electrical current.
How They Work
The operation of concentration cells is fascinating, relying on the natural tendency of ions to move from areas of high concentration to low concentration. This movement creates a potential difference between the cell’s two halves, prompting electrons to flow through the external circuit to equalize the potential. Key steps include:
- Dissolution of ions from the electrode into the more dilute solution.
- Transfer of electrons through the external circuit from the more concentrated side to the less concentrated side.
- Equilibration of ion concentration across the cell, eventually stopping the electron flow.
Key Characteristics
Concentration cells are unique for several reasons:
- Same Electrode Material: Unlike other electrochemical cells, both electrodes are made from the same substance.
- Energy Source: The gradient in ion concentration serves as the energy source, not a chemical reaction.
- Potential Difference: This gradient creates a potential difference that drives electron flow.
Applications
Concentration cells have diverse applications, particularly where measuring, controlling, or utilizing ion concentration gradients is crucial. They’re used in:
- Sensors: For detecting concentrations of gases or ions.
- Metal Recovery: In processes designed to recover metals from dilute solutions.
- Corrosion Prevention: As part of systems to protect against metal corrosion in ships and pipelines.
Chemical Cells
Definition
Chemical cells, often referred to as galvanic cells or voltaic cells, are electrochemical cells that generate electrical energy through spontaneous redox reactions between two different materials, typically metals. The essence of these cells is the chemical difference between the electrodes.
Operation Principle
The magic of chemical cells lies in the chemical reaction between the electrode materials and the electrolyte. These steps outline the process:
- Oxidation occurs at the anode, where electrons are lost.
- Reduction takes place at the cathode, where electrons are gained.
- The flow of electrons through the external circuit generates electrical energy.
This sequence of events harnesses the chemical energy stored in the reactants, converting it into usable electrical energy.
Key Features
Chemical cells are characterized by several key features:
- Different Electrode Materials: Each electrode is made from a different substance, facilitating distinct oxidation and reduction reactions.
- Spontaneous Reactions: The redox reactions that generate electricity occur spontaneously.
- Electrical Energy: The main output is electrical energy, derived from the chemical potential energy of the electrodes and electrolyte.
Uses
The versatility of chemical cells allows for their use in a wide array of applications, such as:
- Batteries: Powering everything from remote controls to electric vehicles.
- Power Generation: In larger systems like fuel cells for clean energy solutions.
- Portable Electronics: Ensuring the mobility and convenience of modern gadgets.
Key Differences
Electrode Reactions
Contrast between Electrode Reactions in Concentration and Chemical Cells
In concentration cells, both electrodes are made of the same material and immerse in electrolytes of different concentrations. The reaction is driven by the concentration gradient, leading to the movement of ions to equalize concentrations across the cell. This creates a potential difference and, consequently, an electric current without the need for different electrode materials.
In contrast, chemical cells utilize electrodes made of different materials, which participate in distinct chemical reactions. The anode undergoes oxidation, losing electrons, while the cathode undergoes reduction, gaining electrons. These spontaneous redox reactions between the electrodes and the electrolyte generate the cell’s electrical energy.
Energy Generation
How Energy is Generated Differently
Concentration cells generate energy purely from the concentration difference of the electrolyte between the two half-cells. This process is an elegant demonstration of how potential energy inherent in concentration gradients can be converted into electrical energy.
Chemical cells, on the other hand, derive their energy from the chemical potential energy stored in the different materials of the electrodes. The electrochemical reaction between the anode, cathode, and electrolyte transforms this chemical potential into electrical power.
Application Areas
Diverse Applications and Their Relevance
Concentration cells have found their niche in precision applications where the detection or measurement of ion concentrations is crucial, such as in electrochemical sensors, metal recovery, and corrosion prevention technologies.
Chemical cells are the backbone of the battery industry, powering everything from portable electronics to electric vehicles. Their ability to store and deliver energy efficiently makes them indispensable in energy storage and power generation fields.
Similarities
Despite their differences, concentration and chemical cells share several core similarities:
- Both types of cells operate on the principle of redox reactions to generate electrical energy.
- Electrolytes play a crucial role in both cells, facilitating the movement of ions necessary for the flow of current.
- They both contribute significantly to the advancement of energy technologies, showcasing the versatility and importance of electrochemical systems.
Significance in Technology
Energy Solutions
Both concentration and chemical cells offer unique energy solutions. Concentration cells are key in applications where energy generation from concentration differences can be utilized efficiently, such as in sensing technologies. Chemical cells, with their ability to store and deliver power on demand, are fundamental in portable and stationary energy storage systems, driving forward the possibilities of renewable energy integration and electric mobility.
Environmental Impact
The environmental impact of these cells varies. Concentration cells, by their nature, can be seen as more sustainable, especially if they use non-toxic materials and saline solutions. Their application in corrosion prevention and metal recovery also contributes to environmental protection.
Chemical cells, particularly in battery technology, face challenges related to material sourcing, recycling, and disposal. However, advances in battery chemistry and recycling technologies are addressing these issues, aiming to minimize the environmental footprint of chemical energy storage solutions.
Future Prospects
Research and Development
Current trends in the research of electrochemical cells focus on increasing efficiency, reducing costs, and minimizing environmental impact. For concentration cells, research aims to exploit novel materials and ion-selective membranes to enhance their applicability. In chemical cells, significant efforts are directed towards next-generation battery technologies such as solid-state batteries, lithium-sulfur, and flow batteries, promising higher energy densities and better safety profiles.
Potential Innovations
The future innovations in electrochemical cells could revolutionize how we store and use energy, with implications for technology and the environment. For instance, advancements in concentration cell technology could lead to more efficient sensors and energy harvesting devices, exploiting environmental gradients for power generation. In chemical cells, breakthroughs could provide safer, more sustainable, and higher capacity batteries, propelling the shift towards renewable energy sources and supporting the global push for electrification of transportation and grid storage solutions.
Frequently Asked Questions
What is an electrochemical cell?
An electrochemical cell is a device that generates electrical energy from chemical reactions or, conversely, uses electrical energy to drive chemical reactions. This dual ability categorizes them into two main types: galvanic cells, which produce electricity through spontaneous reactions, and electrolytic cells, which consume electricity to induce non-spontaneous chemical changes.
How do concentration cells differ from chemical cells?
Concentration cells generate electricity through the difference in concentration of the electrolyte across two half-cells, without relying on different electrode materials or external chemical reactions. In contrast, chemical cells, or galvanic cells, utilize different electrode materials and the spontaneous redox reactions between them to produce electrical energy.
What are the applications of concentration and chemical cells?
Concentration cells are often used in sensors, corrosion prevention, and metallurgical processes, capitalizing on their ability to generate current from concentration differences. Chemical cells find widespread application in batteries, powering everything from portable electronics to electric vehicles, due to their efficient energy conversion from chemical to electrical form.
Why are electrochemical cells important in technology?
Electrochemical cells are fundamental to a myriad of technologies due to their efficient conversion between chemical and electrical energy. This makes them indispensable in batteries, fuel cells, and electrochemical sensors, underpinning much of modern technology, from portable electronics to renewable energy systems and medical devices.
Conclusion
The exploration of concentration cells and chemical cells unveils the rich tapestry of electrochemistry and its pivotal role in advancing energy technologies. By harnessing the unique properties of each cell type, scientists and engineers continue to push the boundaries of energy storage, conversion, and efficiency. This journey not only fuels the technological innovations that power our daily lives but also contributes to the sustainable energy solutions critical for our future.
In closing, the understanding and application of these electrochemical cells embody the confluence of science, technology, and innovation. As research advances, so too will the potential applications and efficiencies of these cells, highlighting their enduring importance in the quest for clean, reliable, and efficient energy sources.
