Cohesion and surface tension are fundamental concepts in the study of fluids, with wide-ranging applications from the biological functions of cells to the design of high-tech materials. While often discussed in tandem due to their reliance on similar forces, these two phenomena exhibit distinct behaviors that influence various natural and engineered systems. By examining these concepts, we can gain deeper insights into the molecular interactions that govern the behavior of liquids.
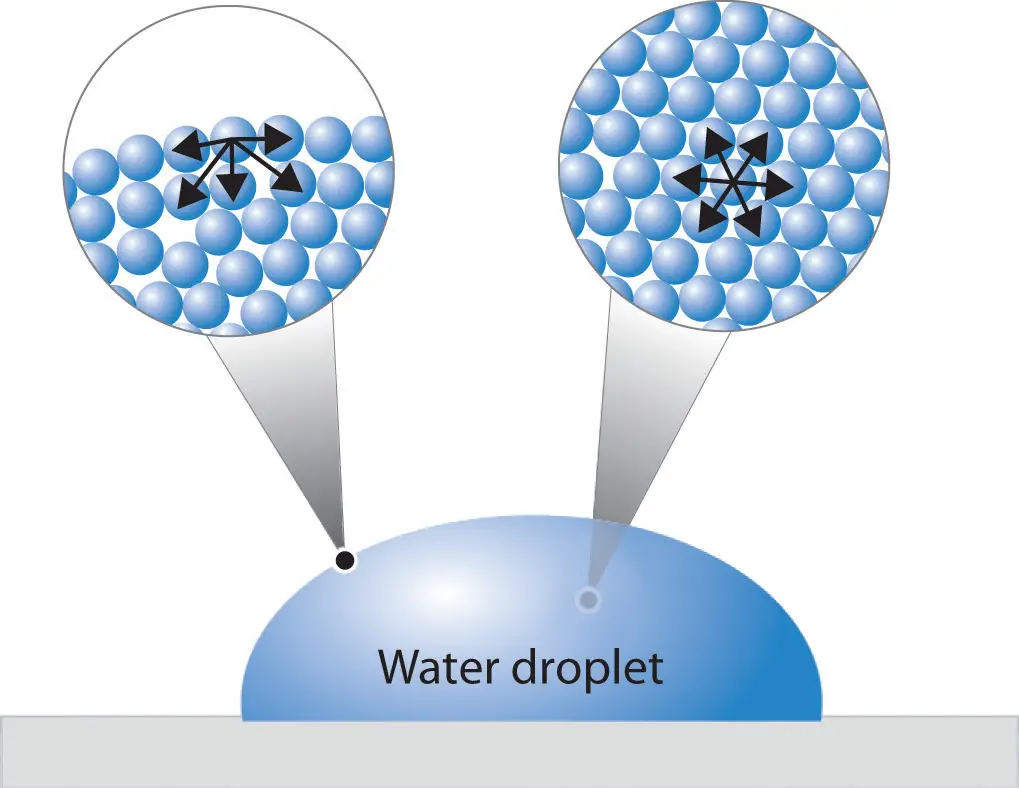
Cohesion refers to the attractive force between like molecules, such as water molecules sticking together in a droplet. Surface tension, on the other hand, is the result of cohesive forces among liquid molecules at the surface of a liquid that are not surrounded by similar molecules on all sides, leading to a contraction of the surface. This distinction explains why water can form beads on a smooth surface and why insects can walk on water.
Both cohesion and surface tension play critical roles in everyday phenomena and technological processes, influencing everything from the way paints spread and dry, to how insects skitter across ponds. Their effects are omnipresent in our daily lives, shaping both the natural world and human-made environments in profound ways.
Basics of Cohesion
Definition and Fundamental Concept
Cohesion refers to the force that holds together molecules of the same substance. This internal force is especially prominent in liquids such as water, where molecules are close enough to exert a strong attractive force on each other. This is what allows substances to resist separation and maintain their structural integrity under various conditions.
Factors Influencing Cohesion
Several factors play crucial roles in determining the strength of cohesion:
- Molecular Structure: The shape and size of molecules can affect how tightly they can pack together, influencing the cohesion.
- Intermolecular Forces: Forces like hydrogen bonds, dipole-dipole interactions, and London dispersion forces directly impact the cohesive properties of a substance.
- Temperature: Higher temperatures tend to decrease cohesion as increased molecular motion can overcome the attractive forces between molecules.
- Purity of the Substance: Impurities can disrupt the uniform intermolecular forces among molecules, thereby reducing cohesion.
Basics of Surface Tension
Definition and Core Principles
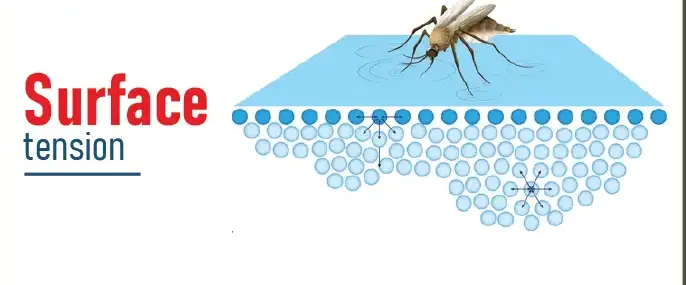
Surface tension is the elastic tendency of liquids which makes them acquire the least surface area possible. This phenomenon occurs because molecules at the surface of a liquid are not surrounded by similar molecules on all sides and thus, they cohere more strongly with those directly associated with them on the surface.
Factors Affecting Surface Tension
Surface tension can be influenced by several external and internal factors:
- Type of Liquid: Different liquids have different surface tension values, primarily due to differences in molecular composition.
- Temperature: As temperature increases, surface tension generally decreases, since the kinetic energy of the molecules increases, reducing the effectiveness of cohesive forces at the surface.
- Presence of Surfactants: Substances like soaps and detergents can reduce surface tension dramatically by interfering with the cohesive forces among surface molecules.
Cohesion at Molecular Level
Molecular Forces Involved
At the molecular level, cohesion is primarily governed by intermolecular forces:
- Hydrogen Bonds: Strong, directional attractions between hydrogen and electronegative atoms like oxygen or nitrogen.
- Van der Waals Forces: Include attractions and repulsions between atoms, molecules, and surfaces, as well as other interactions caused by asymmetrical distribution of electrons within molecules.
Examples from Nature and Technology
In nature, cohesion allows for phenomena like water droplets forming on leaves. In technology, it is exploited in the development of non-drip paints that improve application efficiency and appearance.
Surface Tension Explained
How It Manifests in Liquids
Surface tension manifests as a film on the surface of liquids, capable of supporting light objects like a needle or an insect due to the cohesive forces among the surface molecules.
Real-World Examples
- Water Droplets: Surface tension is responsible for the shape of water droplets on surfaces, which is crucial in various coating and printing technologies.
- Soap Bubbles: These are stabilized by surface tension, which forms spherical shapes to minimize surface area.
Comparing Cohesion and Surface Tension
Similarities Between the Two Phenomena
Both cohesion and surface tension are results of intermolecular forces. They play essential roles in the behavior of liquids and are pivotal in various biological and technological processes.
Key Differences Highlighted
While cohesion refers to the overall attraction between molecules in a substance, surface tension specifically relates to the state of molecules at the surface. This differentiation is crucial in understanding various surface phenomena and their applications in science and engineering.
Impacts on Water Behavior
Water Droplets and Cohesion
Cohesion plays a crucial role in the formation of water droplets. This is because water molecules are strongly attracted to each other due to hydrogen bonding. When water is in contact with air, these cohesive forces pull the molecules together, forming droplets. This behavior can be observed:
- Raindrops: Cohesion helps raindrops maintain their shape as they fall from clouds.
- Condensation: On a cold surface, water vapor condenses into droplets because the cohesive forces draw the water molecules together.
Water Strider and Surface Tension
The water strider, an insect that can walk on water, showcases the effects of surface tension. Surface tension allows these insects to stay atop the water without sinking, thanks to their long, hydrophobic legs that distribute their weight and minimize contact with the water surface. This is an excellent example of how surface tension supports weight and allows movement without breaking the liquid’s surface.
Applications in Technology
Cohesion in Manufacturing
Cohesion is a key factor in various manufacturing processes:
- Adhesives: The effectiveness of glues and other adhesives depends significantly on the cohesive forces within the adhesive material, which must be strong enough to hold materials together.
- Paints and Coatings: Cohesion affects how paint adheres and stays on a surface, as well as how it spreads and dries.
Surface Tension in Devices
Surface tension finds numerous applications in technological devices, especially in:
- Inkjet Printers: Surface tension in the ink allows precise droplet formation, crucial for achieving high-quality prints.
- Medical Devices: Microfluidic devices, which handle small volumes of fluids, rely on surface tension to transport and mix chemicals in labs on a chip.
Everyday Examples
Cohesion in Household Products
Cohesion influences the performance of many household products:
- Cleaning Agents: Water’s cohesive properties help cleaning agents spread uniformly over surfaces.
- Cooking: Syrups and oils exhibit cohesion, affecting their texture and the way they interact with other ingredients.
Surface Tension in Daily Activities
Surface tension is a part of everyday life, noticeable in:
- Washing and Cleaning: Water forms droplets on surfaces, influenced by surface tension, affecting how water cleans and rinses away dirt.
- Gardening: Water beads on leaves demonstrate surface tension, affecting how plants shed water and dew.
Challenges and Solutions
Issues Caused by Cohesion and Surface Tension
Cohesion and surface tension can also pose challenges:
- Pipe Flow: In pipelines, especially those carrying oil or natural gas, cohesion can cause clogs and slow down the flow of fluids.
- Breathing Difficulties: In the medical field, respiratory therapies sometimes struggle with the cohesive properties of bodily fluids.
Innovative Solutions in Industry
Industries have developed innovative solutions to address these challenges:
- Surfactants: Chemicals that reduce surface tension can prevent clogs in pipes and help in respiratory treatments by making fluids less cohesive.
- Coating Technologies: Advances in non-stick coatings and hydrophobic materials help manage surface tension and cohesion, improving everything from cookware to waterproof clothing.
Frequently Asked Questions
What causes cohesion?
Cohesion is primarily caused by intermolecular forces such as hydrogen bonding, particularly prevalent among water molecules. These bonds make the molecules attract one another, creating a consistent and stable structure.
How does surface tension work?
Surface tension occurs due to the cohesive forces among molecules at the surface of a liquid, which experience a net inward force since they are only surrounded by like molecules on the liquid side. This creates a tension on the surface, acting like a stretched elastic membrane.
Why is surface tension important?
Surface tension is crucial for various biological and physical processes, such as the ability of small organisms to walk on water and the formation of droplets. It also influences technological applications including inkjet printing and microfluidic devices.
Can surface tension be altered?
Yes, surface tension can be altered by changing the composition of the liquid, temperature, or by adding surfactants, which decrease the cohesive forces at the surface of liquids, thus reducing the surface tension.
How do cohesion and surface tension affect the environment?
Cohesion and surface tension affect natural water systems and influence processes like raindrop formation and the capillary action that helps plants transport water from their roots to leaves.
Conclusion
Cohesion and surface tension are integral to understanding a wide array of both natural phenomena and engineered solutions. Their roles extend from the simple act of forming raindrops to complex applications in medical technologies and environmental science. As we continue to explore and manipulate these forces, new innovations and more efficient solutions are likely to emerge.
In sum, recognizing the nuances between cohesion and surface tension not only enriches our comprehension of physical sciences but also enhances our ability to innovate across various disciplines. These concepts underscore many everyday interactions and offer endless possibilities for future technological advancements.