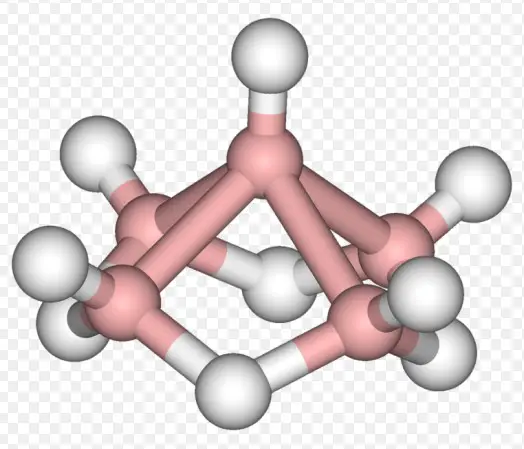
Boranes, compounds of boron and hydrogen, represent a fascinating class of chemicals due to their unique structures and diverse applications in the field of chemistry. The complexity and utility of these compounds are enhanced further when we consider the different types of boranes, namely closo, nido, and arachno boranes. Each type exhibits distinct structural properties that influence their reactivity and potential applications.
Closo, nido, and arachno boranes are distinguished by their structural frameworks and bonding characteristics. Closo boranes possess closed, cage-like structures, nido boranes have open, nest-like frameworks, and arachno boranes feature even more open structures that resemble spiders’ webs. These structural differences are crucial for chemists to understand as they dictate the compounds’ stability, reactivity, and utility in various chemical processes.
The study of these boranes opens up avenues for advancements in material science, catalysis, and organic synthesis, among other fields. Their ability to form complex structures from relatively simple building blocks of boron and hydrogen showcases the versatility and potential of chemical compounds. As we explore the distinctions between closo, nido, and arachno boranes, we uncover the depth of complexity within chemical science, revealing insights into molecular geometry, electron distribution, and bond formation.

Boranes Explained
Historical Background
Boranes, compounds formed from boron and hydrogen, have a rich history that dates back to the early 19th century. Initially, their discovery was met with curiosity due to their unusual properties and structures. Over the years, chemists have unraveled the complexity of boranes, leading to groundbreaking applications in chemistry and materials science.
The development of boranes in chemistry has been marked by significant milestones, including the identification of their unique bonding patterns and the classification of their diverse structures. These achievements have provided deep insights into the world of inorganic chemistry, paving the way for innovative research and technological advancements.
Structural Basics
Boranes are characterized by their boron-hydrogen bonding. The simplest borane, diborane (B2H6), serves as a foundational compound from which more complex boranes are derived. Boranes exhibit a range of structures, from simple to highly complex, influenced by the number of boron and hydrogen atoms and their arrangement.
The structure of boranes is crucial in determining their chemical behavior. This includes their reactivity, stability, and ability to form bonds with other atoms. Understanding the molecular structure of boranes is key to unlocking their potential in various chemical processes.
Closo Boranes
Definition and Characteristics
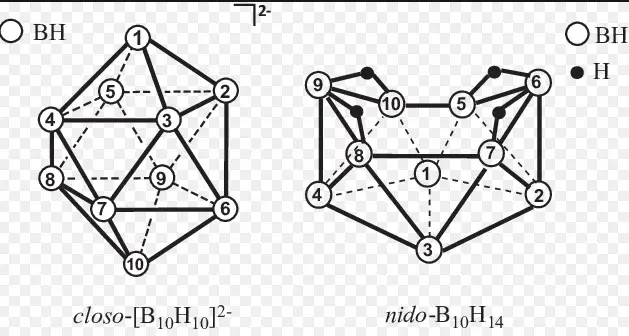
Closo boranes are a type of borane with closed, cage-like structures. They are notable for their high stability and symmetrical geometric shapes. These compounds typically follow the formula B_nH_n^2+, where n is the number of boron atoms, indicating a closed polyhedral structure.
Structural Features
The geometric structure of closo boranes is fascinating. They resemble geometric solids, including triangles, tetrahedrons, and other polyhedra, depending on the number of boron atoms. This closed cage structure is responsible for their unique properties, including their stability and reaction pathways.
Applications
Closo boranes find applications in chemical synthesis and materials science. They are used as catalysts in organic reactions and in the creation of boron-based materials with exceptional hardness and thermal stability. Their predictable structures and reactivity make them valuable tools in the chemist’s toolkit.
Nido Boranes
Definition and Characteristics
Nido boranes are characterized by their open, nest-like structures. Unlike closo boranes, nido boranes have one less vertex, following the general formula B_nH_n+4. This structure gives them unique chemical properties, including reactivity that differs significantly from their closo counterparts.
Structural Features
Nido boranes’ open-framework structures are less symmetrical than closo boranes. Their structure resembles a nest or basket, with boron atoms forming the base and hydrogen atoms capping the structure. This openness leads to different bonding configurations and reactivities.
Applications
In research and industry, nido boranes are utilized for their reactive properties. They serve as intermediates in the synthesis of other boron compounds and as reagents in organic synthesis. Their ability to act as Lewis acids makes them useful in catalysis and polymerization processes.
Arachno Boranes
Definition and Characteristics
Arachno boranes are distinguished by their even more open, web-like structures. Following the formula B_nH_n+6, these compounds feature a framework that extends beyond the nido structure, offering even more complex bonding possibilities.
Structural Features
The structure of arachno boranes is marked by its complexity, resembling a spider’s web. This extended openness allows for additional bonding sites, making arachno boranes highly reactive and versatile in chemical reactions.
Applications
Arachno boranes are employed in a variety of chemical processes. Their reactivity is harnessed in organic synthesis, where they can add boron atoms to organic molecules, facilitating the creation of new compounds. Additionally, their unique properties are explored in the development of new materials and as catalysts in chemical transformations.
Comparative Analysis
Closo vs. Nido
Closo and nido boranes differ significantly in structure and applications. Closo boranes, with their closed, cage-like structures, are highly stable and less reactive than nido boranes. This stability stems from their symmetrical geometric shapes, which effectively distribute electron density and minimize reactive sites. In contrast, nido boranes have open, nest-like structures that expose more reactive sites, making them more chemically active.
From an application perspective, the stability of closo boranes makes them ideal for use in environments where chemical stability is paramount, such as in the formation of inert coatings or as catalysts in certain types of chemical reactions. Nido boranes, with their higher reactivity, are often employed as intermediates in the synthesis of other boron-containing compounds and in organic synthesis, where their open structure can facilitate the addition of other elements or compounds.
Nido vs. Arachno
Nido and arachno boranes share some similarities due to their open structures but differ in the degree of openness and resulting reactivity. Nido boranes are like a partially opened basket, providing a moderate level of reactivity and versatility in chemical reactions. Arachno boranes, with an even more open structure resembling a spider’s web, offer greater reactivity and more potential bonding sites.
This difference in structure leads to variations in their applications. Nido boranes’ moderate reactivity makes them suitable for use in specific types of organic synthesis and in the creation of boron clusters. Arachno boranes, given their high reactivity, find use in more specialized chemical reactions, particularly in the synthesis of complex organic compounds where additional reactive sites can be beneficial.
Closo vs. Arachno
The comparison between closo and arachno boranes highlights the spectrum of stability and reactivity in borane chemistry. Closo boranes are the epitome of stability among boranes, with their closed structures minimizing reactivity and making them suitable for applications requiring stable, inert materials. Arachno boranes sit at the other end of the spectrum, with their extended, web-like structures offering high reactivity and the ability to participate in a wide range of chemical reactions.
The stark differences in their structures underscore the diverse potential applications of these boranes. While closo boranes are often used in stable, long-lasting materials and as catalysts that do not readily decompose, arachno boranes are valuable in dynamic chemical environments where their reactivity can be harnessed to facilitate complex chemical syntheses.
Chemical and Physical Properties
Stability and Reactivity
The stability and reactivity of boranes are directly influenced by their structural characteristics. Closo boranes, being the most stable, resist degradation and remain inert under conditions that would typically promote the reactivity of other chemicals. Nido boranes offer moderate stability but increased reactivity, making them versatile in synthetic chemistry. Arachno boranes, with the least stability, are highly reactive and capable of engaging in a broad array of chemical reactions.
Bonding and Electronic Structure
The bonding and electronic structure of boranes are key to understanding their chemical behavior. Boranes exhibit multicenter bonding, where electrons are shared among several boron atoms, creating a delocalized bonding network. This unique feature contributes to the stability of closo boranes and the reactive nature of nido and arachno boranes. The electronic structure, particularly in arachno boranes, allows for the addition of other elements, providing a pathway to synthesize complex molecules.
Synthesis and Manipulation
Synthesis Techniques
Synthesizing closo, nido, and arachno boranes involves a variety of techniques, often starting with simple boranes like diborane (B2H6) and manipulating them to achieve the desired structure. Common methods include:
- Hydroboration, where hydrogen and boron are combined in the presence of a catalyst.
- Pyrolysis, involving the decomposition of a borane precursor to form a more complex borane.
- Stepwise synthesis, where additional boron and hydrogen atoms are gradually added to build the desired borane structure.
Challenges in Synthesis
The synthesis of complex boranes presents several challenges, including:
- Controlling reactivity, to prevent unwanted side reactions.
- Achieving desired structures, which requires precise conditions and often yields low.
- Handling and storage, as some boranes are highly reactive and require special conditions to remain stable.
Future Prospects
Technological Innovations
The future of closo, nido, and arachno boranes in technological innovations is promising. Their unique structures and properties open up possibilities in creating new materials with enhanced features, such as increased hardness, thermal stability, or specific catalytic activities. Additionally, their role in organic synthesis could lead to the development of novel pharmaceuticals and complex organic molecules.
Research Directions
Emerging research areas include the exploration of boranes in energy storage, where their high hydrogen content could be utilized in hydrogen storage technologies. Another area is the use of boranes in nanotechnology, where their ability to form structured clusters can contribute to the development of nanoscale materials and devices.
Frequently Asked Questions
What are Boranes?
Boranes are compounds composed of boron and hydrogen. They are known for their unique structures and have applications ranging from chemical synthesis to materials science. Boranes can form complex, three-dimensional shapes, making them integral to studying molecular geometry and chemical bonding.
How do Closo, Nido, and Arachno Boranes differ?
The primary difference lies in their structures. Closo boranes have closed, cage-like structures, making them relatively stable. Nido boranes, with open, nest-like frameworks, and arachno boranes, featuring even more extended spider-web-like structures, display varying degrees of reactivity and stability. These structural differences significantly affect their chemical properties and applications.
Why are Boranes important in Chemistry?
Boranes are crucial in chemistry for several reasons. Their diverse structures and bonding configurations make them excellent subjects for studying theoretical chemistry principles. Additionally, boranes are used in organic synthesis, materials science, and as catalysts in various chemical reactions, highlighting their practical importance beyond academic interest.
Can Boranes be synthesized?
Yes, boranes can be synthesized through various chemical reactions, often involving the direct combination of boron with hydrogen under specific conditions. Advanced synthetic techniques also allow for the creation of complex borane structures, including closo, nido, and arachno forms, enabling researchers to study their properties and applications more extensively.
Conclusion
The exploration of closo, nido, and arachno boranes offers a window into the intricate world of chemical structures and their impacts on compound reactivity and utility. These boranes not only underscore the importance of molecular geometry and electron distribution but also highlight the potential of boron and hydrogen compounds in advancing various fields of chemistry. Understanding the distinctions between these types of boranes enriches our knowledge of chemical science, opening doors to new research and applications.
As we continue to uncover the properties and potentials of closo, nido, and arachno boranes, the boundaries of chemical science are expanded, paving the way for innovations in materials science, catalysis, and beyond. The study of these compounds is a testament to the ever-evolving nature of chemistry, a field where the discovery of fundamental differences in structure can lead to significant advancements in technology and industry.