Carbocations are pivotal intermediates in numerous organic reactions, underpinning many processes in synthetic and mechanistic organic chemistry. Their structure, stability, and reactivity dictate the course of a reaction, making their study essential for chemists. At the heart of understanding these species lies the distinction between classical and nonclassical carbocations, each possessing unique characteristics that influence their behavior in chemical reactions.
Classical carbocations are defined by a carbon atom bearing a positive charge and three bonds, making them electron-deficient and highly reactive. Nonclassical carbocations, on the other hand, involve more complex structures with delocalized positive charges, often including bridged intermediates. This fundamental difference affects their stability, formation, and role in organic synthesis, making the distinction crucial for chemists aiming to manipulate chemical reactions effectively.
The distinction between classical and nonclassical carbocations extends beyond mere structural differences. It encompasses their formation mechanisms, the conditions under which each type is stabilized, and their implications in reaction pathways. This delineation not only aids in predicting the outcomes of organic reactions but also in designing new synthetic routes, underscoring the importance of a deep understanding of these intermediates in advancing the field of organic chemistry.

Carbocation Basics
Definition and Formation
Carbocations are positively charged ions with a carbon atom bearing the positive charge. They are critical intermediates in many organic reactions, such as additions, substitutions, and rearrangement processes. Their formation is a crucial step in the reaction mechanism, dictating the direction and outcome of the reaction.
Conditions for formation include the removal of a leaving group from a molecule, leading to the creation of a carbocation. This can happen under acidic conditions or through the homolytic cleavage of bonds during photochemical reactions.
Stability Factors
The stability of a carbocation impacts its reactivity and the type of reactions it can undergo. Several factors influence this stability:
- Electronic factors: The presence of electron-donating groups adjacent to the positively charged carbon can stabilize the carbocation by donating electron density through inductive effects.
- Steric factors: The spatial arrangement of atoms around the carbocation center can affect its stability. Generally, less steric hindrance leads to greater stability.
- Resonance effects: Carbocations that can delocalize their positive charge over multiple atoms through resonance are more stable than those that cannot.
Classical Carbocations
Characteristics
Classical carbocations are characterized by a trivalent carbon atom with six electrons in its valence shell, making it electron-deficient and highly reactive. The electron distribution in classical carbocations is localized to the carbon atom carrying the positive charge.
Examples in Organic Reactions
Classical carbocations play a pivotal role in SN1 reactions, Markovnikov addition of hydrohalic acids to alkenes, and in Friedel-Crafts alkylation reactions.
Stability
Stability of classical carbocations is primarily influenced by:
- Hybridization: Carbocations with sp2 hybridization are more stable than those with sp3.
- Inductive effect: Electron-donating groups near the carbocation center increase stability.
- Hyperconjugation: The delocalization of electrons from adjacent C-H bonds to the empty p-orbital of the carbocation center enhances stability.
Role in reaction mechanisms: Their stability influences the rate and direction of many organic reactions. More stable carbocations lead to more product formation and dictate the preferred pathway in reaction mechanisms.
Nonclassical Carbocations
Characteristics
Nonclassical carbocations are distinguished by their delocalized positive charge across multiple atoms. These often involve bridged structures, where the positive charge is not localized on a single carbon atom but is shared by two or more atoms.
Unique Structure Features
Examples of nonclassical carbocations include the 2-norbornyl cation, where a positive charge is delocalized over a three-carbon bridge, creating a more stable carbocation compared to its classical counterpart.
Stability and Formation
The stability of nonclassical carbocations differs from classical ones due to the delocalization of the positive charge, which lowers the energy of the carbocation.
Conditions favoring their formation include specific structural requirements, such as the presence of a bridging system that allows for charge delocalization, and the participation of solvent molecules in stabilizing the carbocation.
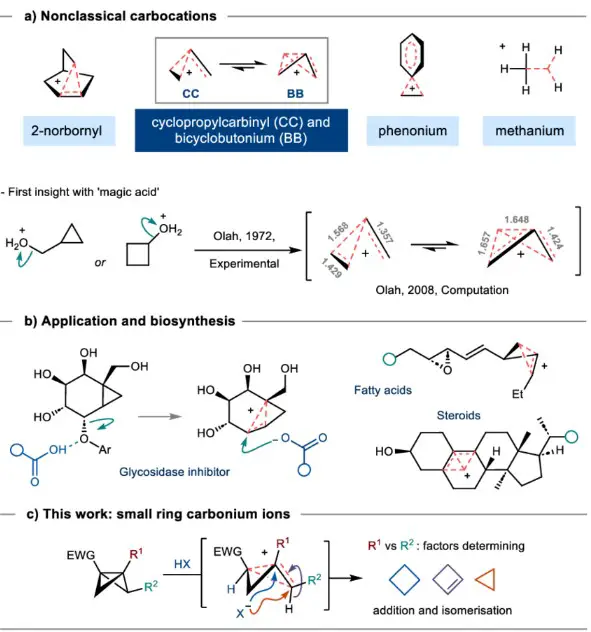
Comparative Analysis
Structural Differences
Classical and nonclassical carbocations differ significantly in their structural configurations. The key difference lies in the distribution of the positive charge and how electrons are delocalized across the molecule.
- Bonding and electron delocalization: In classical carbocations, the positive charge is localized on a single carbon atom. This carbon is sp2 hybridized, forming three sigma bonds and possessing an empty p orbital. Nonclassical carbocations, by contrast, feature a positive charge that is delocalized over several atoms. This delocalization often involves complex bonding scenarios, such as bridged structures, where the charge is shared across a molecular framework.
- Visual representations of structures: Classical carbocations are typically depicted with a “+” sign on the carbon atom bearing the charge. Nonclassical carbocations, however, may be represented by structures with dashed lines or bonds indicating the delocalization of charge across different parts of the molecule.
Reactivity and Stability
The stability of carbocations significantly influences their reactivity and the types of reactions they can participate in.
- Comparative stability reasons: The stability of nonclassical carbocations is often higher due to the delocalization of the positive charge, which spreads the electron deficiency and reduces the energy level of the molecule. Classical carbocations, with their localized charge, are generally less stable and more reactive.
- Reaction pathways and outcomes: The stability of a carbocation can dictate the pathway a reaction takes. For example, more stable carbocations might lead to products through a more controlled mechanism, while less stable carbocations might react more quickly but less selectively.
Role in Organic Synthesis
Carbocations play a critical role in organic synthesis, serving as intermediates in a variety of reactions.
- Synthesis reactions involving classical carbocations: These include reactions like Friedel-Crafts alkylation, where an alkyl group is introduced into an aromatic ring via a classical carbocation intermediate. Classical carbocations are also pivotal in hydration reactions of alkenes, where water is added across a double bond.
- Synthesis reactions involving nonclassical carbocations: Nonclassical carbocations often participate in cycloaddition reactions and rearrangements where the complexity of the molecule allows for the formation of cyclic structures or rearranged carbon skeletons.
- Comparative utility in synthesis: Nonclassical carbocations offer unique advantages in synthesis due to their stability, allowing for more complex reactions to occur under controlled conditions. Classical carbocations, while less stable, are essential for a wide range of straightforward addition and substitution reactions.
Implications in Chemistry
Mechanistic Insights
Understanding the differences between classical and nonclassical carbocations provides profound mechanistic insights into reaction pathways.
- How understanding both types aids in reaction mechanism elucidation: Knowledge of carbocation stability and structure can help predict reaction outcomes and design new synthetic routes. For example, recognizing that a reaction might proceed through a more stable nonclassical carbocation intermediate can influence the choice of reactants and conditions.
Synthetic Applications
The role of carbocations in synthetic applications underscores their importance in developing new molecules and materials.
- Influence on synthetic strategy and product formation: The choice between pathways that involve classical or nonclassical carbocations can drastically affect the efficiency, yield, and purity of synthesized products. Advanced synthetic techniques often rely on the controlled generation and stabilization of carbocations to steer reactions toward desired outcomes.
Frequently Asked Questions
What are carbocations?
Carbocations are positively charged ions where the charge is localized on a carbon atom. These intermediates are crucial in many organic reactions, particularly those involving rearrangements, substitutions, and additions, due to their electron-deficient nature that drives their reactivity.
How do classical and nonclassical carbocations differ?
The main difference between classical and nonclassical carbocations lies in their structure. Classical carbocations have a carbon atom directly bearing a positive charge and forming three bonds. Nonclassical carbocations feature a delocalized positive charge, often involving more complex, bridged structures that can include multiple atoms.
Why is the stability of carbocations important?
The stability of carbocations significantly impacts the course and outcome of chemical reactions. More stable carbocations lead to more favorable reactions, as they can form more readily and undergo further transformations. Understanding the factors that stabilize carbocations is crucial for predicting reaction mechanisms and designing new synthetic routes.
How does resonance affect carbocation stability?
Resonance stabilizes carbocations by delocalizing the positive charge over multiple atoms, reducing the electron deficiency of the positively charged carbon atom. This delocalization distributes the charge over a larger volume, lowering the energy of the carbocation and making it more stable.
Conclusion
The nuanced differences between classical and nonclassical carbocations are more than just academic distinctions; they are fundamental concepts that influence the direction and outcomes of organic reactions. By understanding these differences, chemists can better predict reaction behavior and develop strategies for synthesizing complex molecules. This knowledge not only enriches our understanding of organic chemistry but also enhances our ability to innovate within the field.
In conclusion, the study of carbocations, both classical and nonclassical, offers invaluable insights into the mechanisms of organic reactions. It paves the way for new discoveries and methodologies in synthetic chemistry, underscoring the importance of continuous exploration and understanding of these reactive intermediates. As we delve deeper into their complexities, we unlock the potential to drive forward the boundaries of chemical science.

