Chromate and dichromate ions are significant chemical species, each bearing distinct chemical properties and applications that play pivotal roles in various industrial processes. These compounds are primarily known for their vibrant colors and are used extensively as corrosion inhibitors, in pigments, and in the tanning of leather. Their significance extends beyond mere coloration, influencing both manufacturing processes and environmental practices.
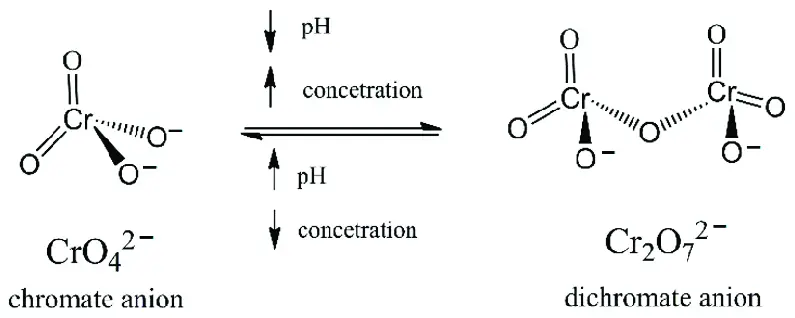
Chromate and dichromate differ primarily in their chemical composition and the conditions under which they transform into each other. Chromates contain the chromate ion, ���42−CrO42−, and are generally observed under alkaline conditions. Dichromates, on the other hand, consist of the dichromate ion, ��2�72−Cr2O72−, and dominate in acidic solutions. This equilibrium between chromate and dichromate is pH-dependent, highlighting a unique interplay that is crucial for their practical applications and environmental impact.
The interconversion between chromate and dichromate is not only a fascinating aspect of inorganic chemistry but also underscores the adaptability and versatility of these compounds in various settings. From their use in electroplating to their role in the production of safety matches, chromate and dichromate ions exemplify how chemical properties can be harnessed to serve specific industrial needs while also presenting challenges in terms of environmental and health safety.
Basic Chemistry
Chromate Overview
Chemical composition and properties
Chromate compounds contain the chromate ion, ���42−CrO42−. These ions are oxidizing agents and are known for their bright yellow color. The key properties of chromate compounds include solubility in water, ability to form insoluble salts with many metal ions, and their use as corrosion inhibitors due to their passivation of metal surfaces.
Common uses and applications
Chromate finds extensive use in various industries. Some of the most common applications include:
- Corrosion resistance: Used in coatings and primers to prevent rust on metal surfaces.
- Pigments: Employed in paints, inks, and plastics for vibrant colors.
- Safety matches: A component of the match head for its oxidizing properties.
Dichromate Overview
Chemical composition and properties
Dichromate ions, represented as ��2�72−Cr2O72−, exhibit strong oxidative characteristics similar to chromate ions but are more potent. These ions are typically seen as orange-red compounds and are highly soluble in water, making them effective in various chemical reactions.
Common uses and applications
Dichromate applications are critical in several fields, including:
- Metal treatment: Used for cleaning and electroplating metals.
- Wood preservation: Acts as a preservative to prevent rot.
- Analytical chemistry: Serves as an oxidizing agent in volumetric analysis.
Chemical Structure
Chromate Structure
Molecular geometry and bonds
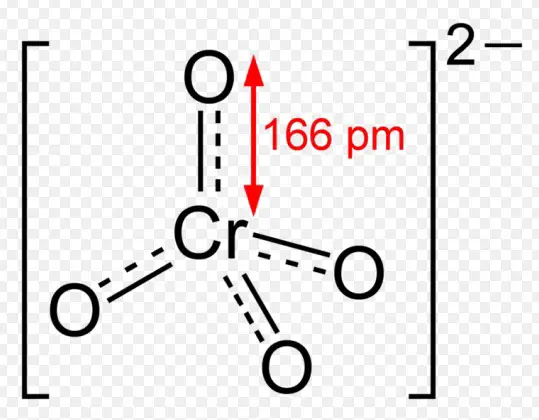
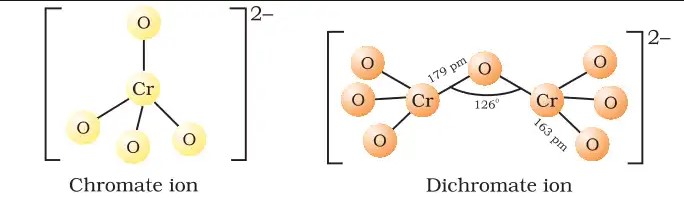
The chromate ion features a tetrahedral geometry with one chromium atom at the center and four oxygen atoms at the corners. The chromium-oxygen bonds exhibit a combination of single and double bond characteristics due to resonance, contributing to the stability and color of the compound.
Dichromate Structure
Molecular geometry and bonds
Dichromate ions display a more complex structure with two chromium atoms connected through an oxygen bridge. Each chromium is also bonded to three other oxygen atoms, forming a somewhat distorted tetrahedral shape. This configuration leads to strong oxidative properties.
Formation and Conversion
Formation Process
Chromate and dichromate are formed through the oxidation of chromium compounds, typically starting from chromium(III) oxide or other chromium(III) salts. The process involves:
- Oxidation: Chromium(III) compounds are oxidized to chromium(VI), usually by an oxidizing agent.
- Solution adjustment: The pH of the solution determines the predominance of chromate or dichromate ions.
Conversion Between Chromate and Dichromate
Conditions for conversion
The balance between chromate and dichromate depends on the pH level of the solution:
- Alkaline conditions favor the formation of chromate ions.
- Acidic conditions shift the equilibrium towards dichromate ions.
Environmental factors affecting the equilibrium
Factors such as temperature and the concentration of other ions in the solution can also influence this equilibrium. Understanding this balance is crucial for industrial applications where specific forms are required.
Applications and Uses
Industrial Applications
Role in manufacturing and industry
Chromate and dichromate ions are vital in numerous industrial processes. They are used for:
- Corrosion protection: Chromate coatings provide long-lasting protection for metal surfaces.
- Plating: Dichromate is used in electroplating to improve adherence and protect the base metal.
- Dyes and pigments: Both compounds are used to create a range of colors for materials.
Environmental Impact
Benefits and concerns
While chromate and dichromate compounds have significant industrial benefits, they also pose environmental and health concerns due to their toxicity and carcinogenic nature. Proper handling, disposal, and the development of safer alternatives are ongoing challenges in their use.
Health and Safety
Exposure Risks
Health hazards associated with chromate and dichromate
Chromate and dichromate compounds pose significant health risks due to their toxic and carcinogenic properties. Exposure can occur through inhalation, skin contact, or ingestion, leading to:
- Respiratory problems: Inhalation of dust or aerosols can cause lung cancer and damage to the nasal septum.
- Skin and eye irritation: Direct contact can result in dermatitis, ulcers, and eye damage.
- Other health issues: Long-term exposure may lead to liver and kidney damage.
Safety Measures
Handling and storage guidelines
Minimizing the risks associated with chromate and dichromate involves:
- Personal Protective Equipment (PPE): Use gloves, goggles, and respiratory protection.
- Proper storage: Store in cool, dry places away from incompatible substances.
- Emergency procedures: Have protocols for spills, exposure, and disposal.
Analytical Methods
Detection of Chromate and Dichromate
Techniques and tools for identification
Identifying chromate and dichromate in samples is crucial for monitoring and regulation. Techniques include:
- Colorimetric tests: Simple, based on the color change when reacting with specific reagents.
- Spectrophotometry: Measures the intensity of color in solutions to quantify chromate or dichromate.
- Ion chromatography: Separates ions for detailed analysis.
Quantitative Analysis
Methods for measuring concentration levels
Quantifying these compounds accurately is essential for environmental and health assessments. Methods include:
- Atomic absorption spectroscopy (AAS): Detects metal ions in samples.
- Inductively coupled plasma mass spectrometry (ICP-MS): Highly sensitive technique for trace metal analysis.
- Voltammetry: Electrochemical method useful in detecting low concentrations.
Environmental and Regulatory Aspects
Environmental Concerns
Impact on ecosystems and water bodies
Chromate and dichromate are environmental pollutants that can have detrimental effects on ecosystems:
- Aquatic life: Toxic to fish and microorganisms, leading to biodiversity loss.
- Soil contamination: Affects plant growth and can enter the food chain.
- Water pollution: Compromises water quality, affecting both wildlife and human health.
Regulatory Standards
Guidelines and limits for usage and disposal
To mitigate environmental and health impacts, regulations govern the use and disposal of chromate and dichromate:
- International standards: Set by bodies like the EPA and WHO, outlining permissible levels in air, water, and soil.
- Disposal practices: Guidelines for safe disposal and treatment of waste containing these compounds.
- Use restrictions: Limitations on use in consumer products and industries.
Recent Advances
Technological Developments
Innovations in chromate and dichromate applications
Research focuses on safer and more sustainable uses of chromate and dichromate:
- Greener alternatives: Development of non-toxic coatings that replicate the corrosion resistance of chromates.
- Advanced treatment methods: New techniques for removing these compounds from industrial waste and contaminated sites.
- Enhanced analytical tools: Improved methods for detecting and quantifying at lower levels.
Research Trends
Ongoing studies and future prospects
The future of chromate and dichromate research aims at reducing their environmental footprint while maintaining their industrial benefits:
- Bioremediation: Exploring natural processes to detoxify contaminated sites.
- Nanotechnology: Utilizing nanomaterials for effective filtration and remediation.
- Policy and regulation: Evolving standards to keep pace with scientific understanding and technological advancements.
Frequently Asked Questions
What is the chemical difference between chromate and dichromate?
Chromate ions are represented as ���42−CrO42−, featuring one chromium atom bonded to four oxygen atoms. Dichromate ions, symbolized as ��2�72−Cr2O72−, consist of two chromium atoms linked to seven oxygen atoms. The difference lies in their atomic structure and the number of chromium and oxygen atoms involved.
How do chromate and dichromate affect the environment?
Both chromate and dichromate are considered environmental pollutants due to their toxicity. They can infiltrate water bodies, posing significant risks to aquatic life and potentially entering the human food chain. Their disposal and the environmental degradation they cause are subjects of regulatory control and scientific research aimed at minimizing their impact.
Can chromate convert to dichromate and vice versa?
Yes, chromate can convert to dichromate and vice versa through a reversible chemical reaction that is dependent on the pH level of the solution. In alkaline conditions, dichromate ions convert to chromate ions, while in acidic conditions, chromate ions transform into dichromate ions. This conversion is pivotal in various chemical processes and environmental considerations.
Why are chromate and dichromate important in industry?
Chromate and dichromate are integral to many industrial applications, including manufacturing, corrosion prevention, and pigment production. Their unique chemical properties make them suitable for use in metal treatments, leather tanning, and even in safety matches, showcasing their versatility and essential role in modern manufacturing and production processes.
Conclusion
The intricate balance between chromate and dichromate underscores the complexity and beauty of chemical reactions and their practical implications. These compounds are more than just elements on the periodic table; they are active participants in processes that affect our daily lives, from the durability of the items we use to the quality of the environment we live in. Their study not only enriches our understanding of chemistry but also highlights the ongoing need for responsible management and innovation in their application.
As we continue to explore and harness the potential of chromate and dichromate, the conversation around their use, environmental impact, and safety measures remains crucial. It is a testament to the ever-evolving nature of science and technology, where understanding and innovation go hand in hand to address both the challenges and opportunities presented by these remarkable chemical entities.