Luminescence, a phenomenon where light is emitted by a substance without combustion or perceptible heat, intrigues scientists and researchers across various fields. This radiant form of energy release is pivotal in numerous applications ranging from medical diagnostics to environmental monitoring. Two notable forms of luminescence are chemiluminescence and electrochemiluminescence, each with unique mechanisms and uses.
Chemiluminescence occurs when a chemical reaction produces light, while electrochemiluminescence involves the generation of light through electrochemical reactions. The primary difference between these two is the initiation process: chemiluminescence is triggered chemically, whereas electrochemiluminescence is initiated by electrical impulses. This fundamental distinction affects their applications, efficiency, and technological development.
Both chemiluminescence and electrochemiluminescence play crucial roles in scientific advancements and practical applications. Their deployment in high-sensitivity assays, such as those used in clinical diagnostics, showcases their importance in today’s technology-driven world. Understanding their mechanisms and distinctions not only benefits scientific research but also enhances their application in real-world scenarios.
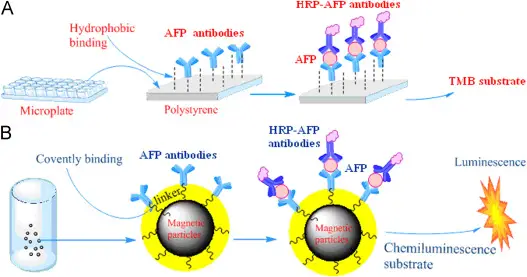
Chemiluminescence Explained
Basics of Chemiluminescence
Definition and Process
Chemiluminescence is a form of luminescence that results from a chemical reaction where light, but not heat, is produced. This phenomenon occurs when molecules transition from a high-energy state to a lower-energy state, emitting photons in the process. Unlike fluorescence, no external light source is required to initiate the reaction.
Key Chemical Reactions
The typical chemiluminescence reaction involves two primary steps:
- Peroxide Formation: A common precursor in these reactions is hydrogen peroxide, which reacts with a suitable luminescent substrate.
- Energy Release: The reaction results in an unstable intermediate that decomposes, releasing energy in the form of light.
For instance, the reaction between luminol and hydrogen peroxide, catalyzed by iron or copper, is a well-known example that produces a distinctive blue glow.
Applications
Use in Biological Assays
Chemiluminescence is extensively used in biological assays, notably in:
- ELISA Tests: For detecting and quantifying proteins or hormones.
- DNA Sequencing: As a method to visualize the presence of specific genetic material.
Industrial Applications
In the industrial sector, chemiluminescence is applied in:
- Safety Testing: Checking for the presence of blood traces or sanitization effectiveness.
- Quality Control: Monitoring oxidation levels in foods and beverages to ensure product stability.
Advantages
Benefits in Various Fields
Chemiluminescence offers several benefits across multiple disciplines:
- High Sensitivity: It can detect very low concentrations of substances.
- Non-Destructive Testing: It does not damage the sample being tested.
Environmental Impact
This technology is considered environmentally friendly as it:
- Reduces Waste: It often requires fewer reagents and produces less waste.
- Uses Less Energy: No additional energy source is needed beyond the chemical reaction.
Limitations
Common Challenges
Some challenges associated with chemiluminescence include:
- Reagent Instability: Some chemicals used are unstable and require specific storage conditions.
- Limited Wavelength Range: The light emitted may not always be within an easily detectable range.
Limitations in Use
- Dependency on Chemicals: The need for specific chemicals can limit the method’s applicability in resource-poor settings.
Electrochemiluminescence Explained
Basics of Electrochemiluminescence
Definition and Process
Electrochemiluminescence (ECL) involves producing light through electrochemical reactions. This process occurs at the surface of electrodes when voltage is applied, initiating a series of reactions that generate an excited state and subsequent light emission.
Mechanism Details
The mechanism typically involves:
- Electron Transfer: Reactants at the electrode surface undergo redox reactions.
- Excited State Formation: These reactions form excited state species that emit light upon returning to the ground state.
Applications
Role in Analytical Chemistry
ECL is highly valued in analytical chemistry for:
- Trace Analysis: Detecting low concentrations of metal ions and organic compounds.
- Sensor Development: Creating sensors for environmental monitoring and food safety.
Medical Diagnostics
In medical diagnostics, ECL is crucial for:
- High-Throughput Screening: Used in drug discovery and biomarker detection.
- Disease Diagnosis: Enabling rapid and accurate testing for various diseases.
Advantages
Precision and Sensitivity
ECL stands out for its:
- High Precision: Offers high reproducibility in measurements.
- Exceptional Sensitivity: Capable of detecting substances at very low levels.
Comparison with Other Methods
Compared to other luminescence techniques, ECL:
- Requires Fewer Reagents: Often needs only the substrate and electrode.
- Provides Better Control: The intensity and duration of light emission can be controlled through electrical inputs.
Limitations
Technical Challenges
Challenges with ECL include:
- Instrumentation: Requires sophisticated equipment and expertise.
- Electrode Degradation: Electrodes can deteriorate over time, affecting performance.
Cost Implications
While ECL offers superior performance, it:
- Can Be Costly: Initial setup and maintenance of equipment can be expensive.
- Requires Skilled Personnel: Needs trained technicians for operation and troubleshooting.

Comparative Analysis
Process Comparison
Step-by-step Comparison of Processes
Chemiluminescence and electrochemiluminescence, though similar in name and end result, engage different sequences to produce light:
- Chemiluminescence:
- Chemical initiator reacts with luminescent material.
- Energy transfer occurs, leading to an excited state.
- Light is emitted as the system returns to a ground state.
- Electrochemiluminescence:
- Application of voltage to electrodes.
- Electrochemical reactions at the electrode surface.
- Excited states are produced, followed by light emission.
Reaction Components
- Chemiluminescence often involves peroxides or oxalates and catalysts like copper or iron.
- Electrochemiluminescence primarily uses ruthenium complexes or quantum dots at electrodes.
Efficiency and Sensitivity
Analysis of Efficiency
Electrochemiluminescence generally shows higher efficiency due to the controlled electrical environment, which can optimize the reaction conditions more effectively than the often unpredictable chemical environments in chemiluminescence.
Sensitivity in Detection
Both methods are highly sensitive. Electrochemiluminescence, however, may edge out due to its ability to finely tune the electrical input, enhancing the precision of light emission and thereby improving detectability at even lower concentrations.
Practical Applications
Side-by-side Application Uses
- Medical Diagnostics: Both methods are used, but electrochemiluminescence is favored for its repeatability and control.
- Environmental Monitoring: Chemiluminescence is often used for its simplicity and effectiveness in outdoor settings.
Comparative Effectiveness
In terms of effectiveness, electrochemiluminescence tends to be more versatile and adaptable to various scientific needs due to its electrical control mechanism.
Costs and Accessibility
Cost Comparison
While both technologies are cost-effective, chemiluminescence often requires less sophisticated equipment, making it more accessible initially. Electrochemiluminescence, though initially more expensive, offers lower running costs over time due to minimal chemical use.
Availability and Scalability
Chemiluminescence is widely available and easy to scale in various settings. Electrochemiluminescence, while scalable, requires more infrastructure support due to its electrical components.
Case Studies
Case Study: Medical Diagnostics
Example of Chemiluminescence Use
In an immunoassay for hormone detection, chemiluminescence has allowed for rapid and accurate results, facilitating quicker patient diagnostics and treatment adjustments.
Example of Electrochemiluminescence Use
Electrochemiluminescence has been critical in developing high-throughput assays that can simultaneously test for multiple conditions, thus streamlining laboratory processes and improving patient care through faster and more accurate results.
Case Study: Environmental Monitoring
Chemiluminescence in Environmental Applications
Used in air quality monitoring, chemiluminescence helps detect nitrogen oxides in the atmosphere, providing data crucial for environmental management and policy-making.
Electrochemiluminescence for Pollution Detection
This technology has been applied in water quality testing, where its high sensitivity allows for the detection of trace metals and organic pollutants, significantly impacting pollution control strategies.
Future Directions
Research Trends
Recent Advancements
Recent research has focused on enhancing the sensitivity and stability of both chemiluminescent and electrochemiluminescent systems. For example, the integration of nanomaterials has shown potential to significantly boost the efficiency of light emissions.
Future Research Areas
The exploration of green chemistry approaches in luminescence and the development of portable luminescent devices for on-site applications are anticipated future trends.
Technological Innovations
Emerging Technologies in Both Fields
The advent of flexible and wearable sensors incorporating luminescent technologies promises a new era of personal health monitoring and environmental sensing.
Potential Market Growth
As the demand for non-invasive diagnostics and real-time environmental monitoring increases, the market for advanced luminescent technologies is expected to see substantial growth.
FAQs
What is chemiluminescence?
Chemiluminescence is the emission of light as a result of a chemical reaction without the need for external light sources. This process is commonly employed in various scientific tests and assays, where it serves as a marker for the presence of specific substances.
How does electrochemiluminescence differ?
Electrochemiluminescence involves the production of light through electrochemical reactions. This method is particularly valued in analytical chemistry for its precision and ability to be controlled by electrical variables, making it a versatile tool for quantitative analysis.
What are common applications of chemiluminescence?
Common applications of chemiluminescence include medical diagnostics, such as in immunoassays where it is used to detect the presence of antigens or antibodies, and in environmental monitoring, where it helps in detecting pollutants.
Are these technologies cost-effective?
Both technologies offer cost-effective solutions in their respective fields. Chemiluminescence is widely utilized for its relatively low operational costs, while electrochemiluminescence is favored in scenarios requiring high sensitivity and specificity.
What are the future trends in luminescence technology?
The future of luminescence technology is promising, with ongoing research focusing on enhancing the sensitivity and specificity of luminescent assays. Innovations aim to develop more environmentally friendly and cost-effective luminescent materials, expanding their applicability.
Conclusion
The exploration of chemiluminescence and electrochemiluminescence reveals a significant impact on both scientific research and practical applications. Their continued development and refinement will likely lead to more advanced and efficient technologies. As researchers strive to improve these luminescent methods, their potential to revolutionize fields such as diagnostics and environmental monitoring grows.
These technologies, with their unique properties and applications, exemplify the synergy between chemistry and technology. Their evolving landscape not only enriches our scientific understanding but also enhances the capabilities of various industries, underscoring the importance of luminescence in modern science and technology.

