Nuclear Magnetic Resonance (NMR) spectroscopy stands as a pivotal technique in the realm of chemistry for elucidating the structure of molecules. By exploiting the magnetic properties of certain nuclei, NMR provides detailed information about the arrangement of atoms, helping scientists unlock the mysteries hidden within chemical compounds. The concepts of chemical shift and coupling constant are fundamental to this technique, offering insights into molecular environments and interactions.
The chemical shift in NMR spectroscopy refers to the variation in resonance frequency of a nucleus caused by its electronic environment, typically reported in parts per million (ppm). On the other hand, the coupling constant quantifies the interaction between nuclear spins, revealing the connectivity and distances between atoms. Together, these parameters furnish a comprehensive view of molecular geometry and dynamics, essential for understanding chemical and biological systems.
Diving deeper, chemical shifts allow chemists to identify the types of chemical environments present in a molecule, such as distinguishing between aliphatic and aromatic hydrogens. Coupling constants, meanwhile, provide a window into the relative positions and bond relationships within molecules, enabling the determination of stereochemistry and conformational structure. These two facets of NMR spectroscopy complement each other, delivering a rich tapestry of information that guides researchers in their quest to decipher molecular structures.
Chemical Shift
Basic Concept
Definition and Significance
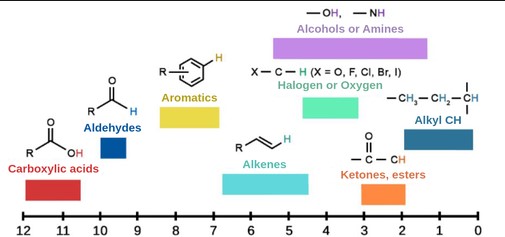
In Nuclear Magnetic Resonance (NMR) spectroscopy, chemical shift is a crucial concept that reflects the resonance frequency of a nucleus relative to a standard reference frequency. It’s measured in parts per million (ppm) and reveals information about the electronic environment surrounding the nucleus. This shift is pivotal because it allows chemists to determine molecular structure and identify functional groups within molecules, making it indispensable in organic chemistry, biochemistry, and medicine.
Factors Influencing Chemical Shift
Several factors can alter the chemical shift in NMR spectroscopy. These include:
- Electron Density: More electron density around a nucleus shields it from the magnetic field, leading to an upfield shift (lower ppm value).
- Electronegative Atoms: Nearby electronegative atoms can deshield a nucleus, causing a downfield shift (higher ppm value).
- Molecular Structure: The overall structure, including bonding and spatial arrangement, can also influence the chemical shift.
Measuring Chemical Shift
Description of the PPM Scale
The ppm scale is a dimensionless unit that represents the chemical shift relative to a reference compound. It’s calculated based on the frequency of the nucleus being observed and allows for the comparison of chemical shifts across different instruments and frequencies.
Techniques for Determining Chemical Shift
To measure chemical shift, chemists follow these steps:
- Prepare the Sample: Dissolve the substance in a suitable solvent, often deuterated to avoid interference.
- Select a Reference Compound: Commonly tetramethylsilane (TMS) due to its inertness and well-defined peak.
- Run the NMR Spectrum: Use an NMR spectrometer to observe the resonance frequencies.
- Calculate Chemical Shifts: Compare the resonance frequencies of the sample to the reference compound and calculate the ppm values.
Applications
Role in Molecular Structure Determination
Chemical shift is key in identifying and characterizing molecular structures. It helps in:
- Determining the identity of functional groups.
- Understanding the spatial arrangement of atoms.
Examples from Organic Chemistry
In organic chemistry, chemical shifts provide insights into:
- Aliphatic vs. Aromatic Hydrogens: Distinct chemical shifts allow for easy differentiation.
- Hydrogen Bonding: Shifts can indicate the presence and strength of hydrogen bonds.
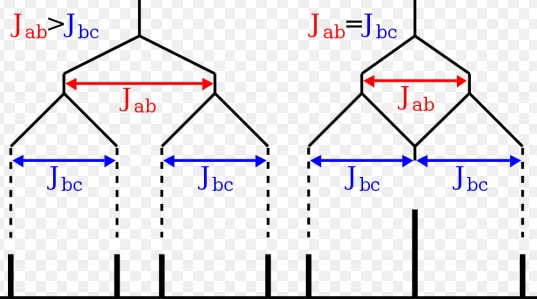
Coupling Constant
Definition
The coupling constant (J) measures the interaction between nuclear spins, influencing the splitting of NMR signals. It’s a critical parameter that offers information on the spatial orientation and distance between atoms, crucial for elucidating molecular geometry.
Explanation of Coupling and Its Significance
Coupling occurs due to the magnetic interactions between adjacent nuclei, leading to the splitting of NMR signals. This phenomenon helps in determining the connectivity and relative positions of atoms within a molecule, enhancing our understanding of its structure.
How Coupling Constants are Measured
Coupling constants are obtained by analyzing the splitting patterns in an NMR spectrum:
- Identify Split Peaks: Look for multiplets indicative of coupling.
- Measure Splitting: Calculate the difference in frequency between the split peaks.
- Report in Hertz: The value is usually given in Hz, independent of the operating frequency of the NMR spectrometer.
Factors Affecting Coupling Constant
Types of Coupling
- J-Coupling: Through-bond interaction, dependent on the bond connectivity.
- Dipolar Coupling: Through-space interaction, relevant in solid-state NMR.
Influence of Bond Angles and Electronegativity
- Bond Angles: Affect the magnitude of J-coupling.
- Electronegativity: Electronegative atoms can alter electron density, influencing J-coupling.
Applications
Insight into Conformational Analysis
Coupling constants provide valuable data on the conformation and flexibility of molecules, aiding in the study of:
- Stereochemistry: Determining cis-trans isomerism and diastereotopic groups.
- Dynamic Processes: Observing conformational changes over time.
Examples in Complex Molecule Studies
In more complex molecules, coupling constants help in:
- Assigning Stereochemistry: Distinguishing between different stereoisomers.
- Structural Elucidation: Providing clues to the three-dimensional structure.
Key Differences
Overview of Differences
While both chemical shift and coupling constant are fundamental to NMR spectroscopy, they serve different purposes. Chemical shift provides information on the electronic environment of nuclei, whereas coupling constant offers insight into the spatial relationship between nuclei.
Comparative Analysis
- Chemical Shift: Indicates the type and environment of atoms.
- Coupling Constant: Reveals the connectivity and orientation of atoms.
Impact on NMR Spectrum Interpretation
Understanding both parameters is crucial for accurately interpreting NMR spectra and deducing molecular structure.
Practical Implications
Decision-making in Spectral Analysis
The combined analysis of chemical shift and coupling constants aids in:
- Identifying Unknown Compounds: Through detailed spectral analysis.
- Determining Molecular Structure: By correlating spectral data with known structures.
Strategy for Complex Structure Elucidation
For complex molecules, a strategic approach involves:
- Sequential Assignment: Using chemical shifts to identify functional groups.
- Detailed Analysis of Coupling Patterns: To deduce the molecular framework.
Advanced Considerations
Chemical Shift Anisotropy
Explanation and Importance in Advanced NMR
Chemical shift anisotropy (CSA) is a phenomenon where the chemical shift of a nucleus varies with the orientation of the molecule in the magnetic field. This variation arises because the electron cloud around a nucleus can shield it differently depending on the molecule’s orientation relative to the external magnetic field. CSA is particularly noticeable in solid-state NMR spectroscopy, where molecules do not tumble rapidly and randomly as they do in solution. Understanding CSA is crucial for accurate interpretation of solid-state NMR spectra, as it provides insights into the shape, orientation, and electronic environment of molecules, enhancing our understanding of material properties and biomolecular structures.
Dynamic NMR Spectroscopy
Role of Temperature and Molecular Dynamics
Dynamic NMR spectroscopy examines how NMR spectra change with temperature and time, offering a glimpse into the molecular dynamics and kinetic processes within a sample. As temperature increases, molecules move more rapidly, affecting both chemical shifts and coupling constants. This movement can lead to the averaging of chemical shift anisotropies and changes in coupling patterns due to conformational changes or chemical reactions. By analyzing these changes, chemists can uncover information about energy barriers, reaction mechanisms, and molecular motions, providing a deeper understanding of chemical and biological systems.
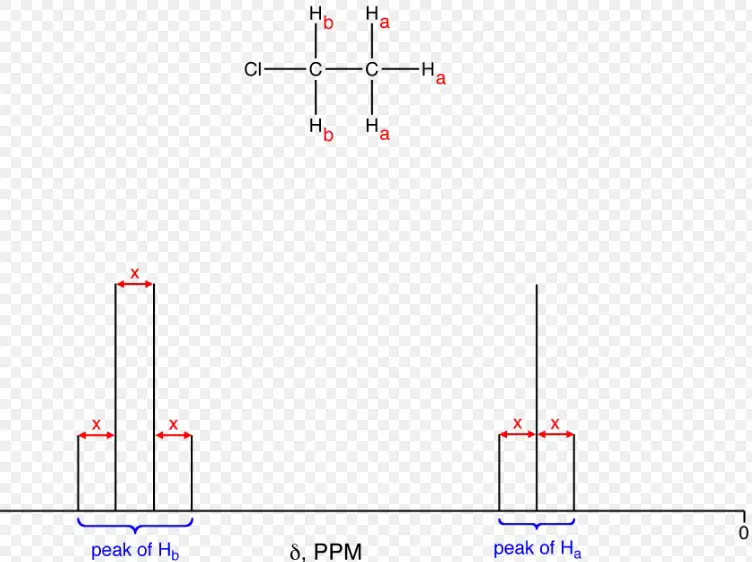
Coupling Patterns
Analysis of Complex Splitting Patterns in NMR Spectra
The analysis of coupling patterns in NMR spectra is a powerful tool for elucidating molecular structures. Complex splitting patterns arise from the interactions between multiple coupled nuclei, with the pattern and intensity of the peaks revealing the number of coupling nuclei, their coupling constants, and the relative arrangement of atoms. By carefully analyzing these patterns, it is possible to deduce the connectivity and geometry of complex molecules, aiding in the identification of unknown compounds and the detailed characterization of molecular structures.
Case Studies
Simple Organic Molecules
Illustration of Chemical Shift and Coupling Constant Roles
One of the foundational applications of NMR spectroscopy lies in the study of simple organic molecules. For instance, the chemical shift can differentiate between methyl (CH3) and methine (CH) groups, while the coupling constant can reveal the presence of cis or trans isomers in alkenes. Consider ethyl acetate, a common solvent and reactant in organic synthesis. Its NMR spectrum showcases distinct chemical shifts for the methyl and methoxy (OCH3) groups, while the coupling constants between the protons in the ethyl group indicate their geminal and vicinal relationships, providing a clear picture of the molecule’s structure.
Biomolecules
NMR Analysis of Proteins and Nucleic Acids
NMR spectroscopy plays a vital role in the study of biomolecules, such as proteins and nucleic acids. These macromolecules present unique challenges due to their size and complexity, but NMR techniques offer unparalleled insights into their structure, dynamics, and interactions. For example, the chemical shifts of amino acid residues in proteins can indicate their involvement in secondary structures like alpha-helices and beta-sheets, while coupling constants can provide information about the conformation of the protein backbone. Similarly, in nucleic acids, NMR can identify base pairing and stacking interactions in DNA and RNA structures. These insights are critical for understanding the molecular basis of biological functions and diseases, leading to advancements in drug discovery and biotechnology.
Frequently Asked Questions
What is a Chemical Shift?
A chemical shift is a measure of the change in resonance frequency of a nucleus in an NMR spectrum, influenced by its electronic environment. This shift, expressed in parts per million (ppm), helps identify the specific type of atoms or groups in a molecule, offering insights into the molecular structure and the electronic surroundings of the nuclei.
How is a Coupling Constant Measured?
The coupling constant, denoted as J, quantifies the strength of interaction between two nuclear spins. It is measured in hertz (Hz) and determined from the splitting patterns observed in NMR spectra. By analyzing these splittings, scientists can deduce the connectivity between atoms and gain insights into the molecule’s spatial arrangement.
Why are Chemical Shift and Coupling Constant Important?
Chemical shift and coupling constant are crucial for interpreting NMR spectra, as they provide valuable information about the molecular structure and dynamics. The chemical shift offers clues about the chemical environment of nuclei, while the coupling constant reveals the relationships between neighboring nuclei. Together, they enable a detailed understanding of molecular geometry and electronic structure.
Can NMR Spectroscopy Identify All Types of Molecules?
NMR spectroscopy is versatile and can identify a wide range of molecules, from small organic compounds to large biomolecules like proteins and nucleic acids. However, its efficacy depends on the presence of nuclei that have a magnetic moment and the complexity of the molecule. For very large or complex molecules, additional techniques and advanced NMR methods may be required to achieve comprehensive structural elucidation.
Conclusion
The intricacies of NMR spectroscopy, particularly through the lens of chemical shift and coupling constant, underscore the sophistication of modern chemical analysis. By providing a detailed snapshot of molecular environments and interactions, these parameters empower chemists and biologists to probe the deepest secrets of matter. The utility of understanding these concepts extends beyond academic curiosity, influencing drug discovery, material science, and the synthesis of novel compounds.
As we continue to refine our understanding of NMR spectroscopy, the roles of chemical shift and coupling constant as fundamental tools in this endeavor cannot be overstated. They not only facilitate a more nuanced comprehension of molecular structures but also pave the way for groundbreaking advancements in science and technology. In this light, the exploration of chemical shift and coupling constant in NMR spectroscopy remains a beacon of progress in the chemical sciences, illuminating the path to discovery and innovation.