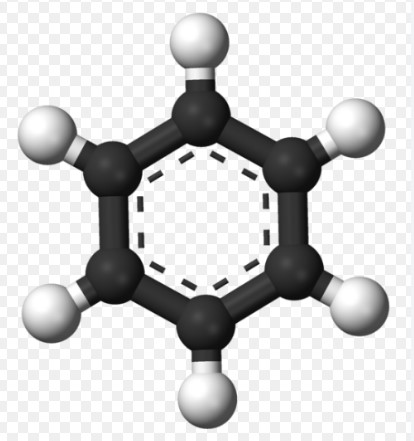
Chemical properties and bonding behaviors like catenation and tetravalency form the cornerstone of understanding complex chemical interactions and the formation of diverse molecular structures. These concepts not only highlight the unique capabilities of elements but also dictate how they interact in various chemical environments. By exploring the foundational elements of chemistry, one can appreciate the intricacies of molecular design and functionality.
Catenation refers to the ability of an element to form bonds with atoms of the same kind, creating chains or rings. Tetravalency, on the other hand, describes an element’s capacity to form four bonds with other elements, significantly impacting the structure and stability of compounds. These properties are crucial in the formation of complex organic molecules and various synthetic materials, influencing everything from biological compounds to industrial polymers.
While catenation is primarily observed in carbon due to its unique electron configuration and bonding capabilities, tetravalency is a broader property seen in several elements including carbon, silicon, and lead. This variance in chemical behavior underpins the formation of an array of substances from simple gases to complex, high-molecular-weight polymers, highlighting the versatility and interdependence of these properties in chemistry.
Catenation Explained
Definition of Catenation
Catenation is the chemical property that allows an element to form bonds with other atoms of the same kind, resulting in a series of connected atoms. This property is crucial in the structure and formation of various chemical compounds, particularly in organic chemistry.
How Catenation Occurs in Elements
Catenation occurs through the sharing of electrons between atoms of the same element, forming strong covalent bonds. The extent and complexity of these bonds are influenced by the element’s electron configuration and its ability to stabilize different bond structures. Elements with a high tendency for catenation often exhibit a unique ability to form stable, multi-atom chains and rings.
Common Elements Exhibiting Catenation
While several elements can catenate, carbon is the most prominent example due to its versatile bonding capabilities. Besides carbon, elements like silicon, sulfur, and phosphorus also show significant catenation ability. Carbon, with its four valence electrons, can form long chains and complex structures such as cyclic and branched molecules, which are foundational to life on Earth.
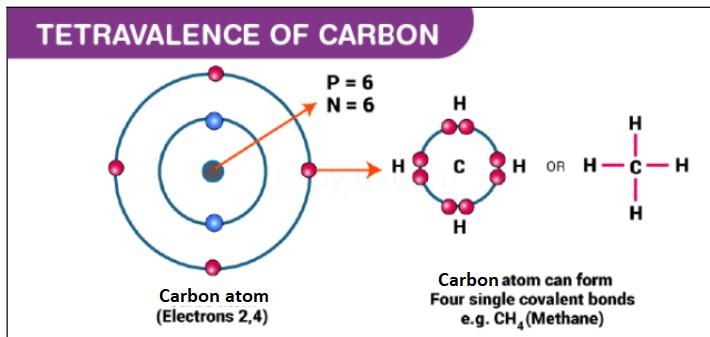
Tetravalency Explained
Definition of Tetravalency
Tetravalency refers to an element’s ability to form four covalent bonds with other atoms. This property is a key factor in determining the chemical structure and reactivity of elements, playing a vital role in the development of complex molecules.
How Tetravalency Functions in Elements
An element is tetravalent if it has four electrons in its outer shell that can be shared with other atoms to form stable covalent bonds. This electron arrangement allows for the formation of a versatile number of compounds due to the ability of the tetravalent atoms to link with multiple other atoms simultaneously.
Examples of Tetravalent Elements
Carbon is the most famous tetravalent element, essential for all known life. Other tetravalent elements include silicon, which is crucial in the semiconductor industry, and lead, used in batteries and protective shielding. These elements can form various stable and complex structures, significantly impacting their applications in different fields.
Comparative Analysis
Structural Differences
Catenation and tetravalency contribute differently to the structural configuration of compounds. While catenation leads to the formation of chains and rings, tetravalency often results in the creation of four distinct covalent bonds that can be oriented in several geometric configurations. This fundamental difference influences the three-dimensional structure and potential chemical complexity of compounds.
Chemical Properties Contrast
Elements that exhibit catenation often form more flexible and variable compounds compared to those that are strictly tetravalent. Tetravalent compounds, however, typically show greater stability and predictability in their chemical properties due to the fixed number of bonds.
Role in Organic Compounds
Both catenation and tetravalency are critical in organic chemistry. Catenation allows for the extensive diversity of organic compounds, as seen in the vast array of carbon-based molecules. Tetravalency, particularly of carbon, provides the structural foundation for these molecules, supporting complex architectures such as rings and branched chains.
Catenation in Organic Chemistry
Catenation in Carbon Compounds
In organic chemistry, the catenation of carbon atoms forms the backbone of most organic molecules, ranging from simple hydrocarbons to complex biomolecules like DNA and proteins. The unique ability of carbon to catenate allows for an almost infinite variety of molecular structures, which are central to biological systems and synthetic materials.
Importance in Molecular Structures
The structural versatility provided by carbon’s catenation is critical for the function and diversity of organic molecules. It influences molecular shape, size, and the three-dimensional arrangement, all of which are crucial for biological activity and material properties.
Examples and Applications
Catenation is seen in the formation of various hydrocarbons, natural and synthetic polymers, and biological macromolecules. Applications range from the use of simple hydrocarbons like methane as fuels to the use of complex DNA molecules in genetic engineering and biotechnology.
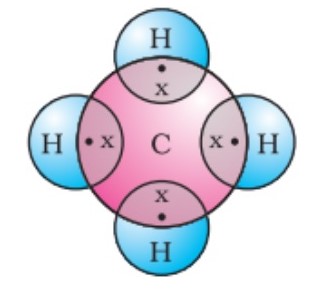
Tetravalency in Organic Chemistry
Impact on Stability and Reactivity
Tetravalency significantly enhances the stability and reactivity of organic compounds. Elements like carbon form four stable covalent bonds, creating a balanced electron configuration that reduces the likelihood of unwanted reactions, which is crucial for the stability of large molecules. This stability is a cornerstone in organic chemistry, influencing everything from the simplest organic compounds to complex biomolecules.
Tetravalency in Carbon and Silicon
Carbon
The tetravalency of carbon is central to its role in organic chemistry. Carbon’s ability to form four covalent bonds allows it to construct a diverse range of complex and stable organic molecules. These include everything from basic hydrocarbons to essential life forms’ molecules like proteins and nucleic acids.
Silicon
Silicon, while less versatile in forming biological compounds, shows its tetravalency prominently in materials science. Its ability to bond with oxygen and other elements forms the basis of silicates and silicones, crucial in various applications from ceramics to medical implants.
Applications in Polymer Chemistry
The role of tetravalent elements, particularly carbon and silicon, is pivotal in polymer chemistry. Polymers, long chains of repeating molecular units, rely on the stable carbon-carbon or silicon-oxygen bonds to form materials ranging from plastics to biocompatible silicones. The predictability and stability imparted by tetravalency allow chemists to engineer polymers with specific properties such as flexibility, strength, and resistance to degradation.
Interaction of Catenation and Tetravalency
Coexistence in Chemical Structures
Catenation and tetravalency often coexist in chemical structures, providing a framework that supports complex molecular architecture. For example, carbon’s ability to catenate, combined with its tetravalency, allows the formation of intricate and stable organic frameworks necessary for both biological and synthetic materials.
Mutual Influence in Compound Formation
The mutual influence of catenation and tetravalency in compound formation cannot be overstated. These properties work synergistically to increase the diversity and complexity of chemical compounds. Catenation extends the backbone of a molecule while tetravalency ensures the addition of different atoms and groups, enhancing the compound’s functional capabilities.
Impact on Chemical Reactivity
The interaction between catenation and tetravalency directly impacts a molecule’s chemical reactivity. Structures that incorporate both properties tend to exhibit unique reactivities, which can be harnessed to create highly specialized chemical reactions and pathways, essential in synthetic chemistry and pharmaceuticals.
Technological and Industrial Applications
Use in Material Science
Tetravalency and catenation find extensive applications in material science. The ability of elements like carbon and silicon to form strong, stable bonds allows for the development of advanced materials such as carbon fiber and silicon carbide, which are used in high-stress environments like aerospace and automotive industries.
Applications in Nanotechnology
In nanotechnology, the precise manipulation of atoms and molecules is key, and here the roles of tetravalency and catenation are crucial. They enable the creation of nanostructures with specific properties, used in electronics, drug delivery systems, and energy storage technologies. Carbon nanotubes and silicon nanoparticles are prime examples, leveraging carbon’s catenation and silicon’s tetravalency.
Importance in Pharmaceutical Chemistry
The pharmaceutical industry relies heavily on the principles of catenation and tetravalency. The design and synthesis of new drugs often involve creating complex organic molecules that require the stability and reactive predictability provided by these chemical properties. From antibiotics to chemotherapy drugs, the ability to engineer molecules with specific interactions within the body is paramount.
Frequently Asked Questions
What is Catenation?
Catenation is the chemical property of an element to form bonds with other atoms of the same element, resulting in the formation of chains or rings. This is most notably seen in carbon, which can create long chains and complex structures vital to organic chemistry.
How does Tetravalency affect chemical bonding?
Tetravalency refers to the ability of an element to form four chemical bonds, which significantly influences molecular structure and stability. This property is essential in the formation of sturdy and stable compounds, particularly in the development of large, complex molecules in organic chemistry.
Why is Carbon unique in its Catenation ability?
Carbon is unique due to its ability to form stable, long chains of atoms through catenation, a property less commonly observed in other elements. This ability is crucial for the development of various organic compounds and is foundational to the study of organic chemistry.
Can Silicon exhibit Catenation?
Yes, silicon can exhibit catenation, but it is less versatile compared to carbon. Silicon atoms can form chains and networks, which are essential in materials science, particularly in the production of ceramics and certain polymers.
Conclusion
The exploration of catenation and tetravalency reveals a fascinating landscape of chemical behavior that underscores the complexity and variety of molecular structures. These properties not only explain the stability and reactivity of compounds but also provide insight into the material capabilities that can be developed from these atomic interactions. As we delve deeper into understanding these characteristics, the potential for new materials and technologies based on these principles continues to expand.