Caros Acid and Marshalls Acid, though not household names, play pivotal roles in the chemical industry, influencing a wide array of manufacturing processes and product developments. Each acid boasts unique properties and applications that set them apart, serving as critical components in the realms of organic synthesis, pollution control, and more. Their significance is underscored by their distinct chemical compositions and the specific roles they fulfill in industrial applications.
Caros Acid, also known as Peroxymonosulfuric acid, is characterized by its potent oxidizing abilities, making it invaluable in various chemical reactions and environmental applications. Marshalls Acid, on the other hand, is known as Peroxydisulfuric acid and distinguishes itself through its ability to act as a stronger oxidizing agent than Caros Acid. While both acids are used to initiate polymerization and as cleaning agents, their differences in reactivity and applications highlight the importance of distinguishing between the two.
Focusing on these acids, it becomes apparent that their chemical structures, production processes, and applications in industry reveal both their versatility and their limitations. From their roles in the synthesis of fine chemicals to their environmental implications, Caros and Marshalls Acids embody the dual nature of chemical compounds as both invaluable tools and subjects of careful handling and consideration.
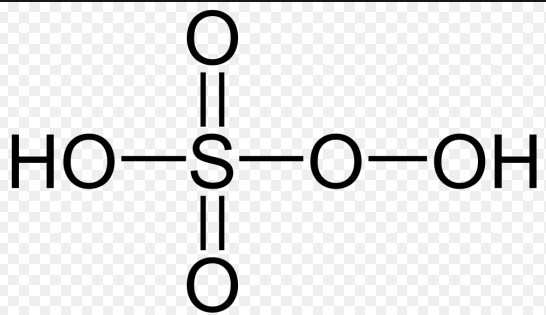
Caros Acid Overview
Definition and Composition
Caros Acid, also known scientifically as Peroxymonosulfuric acid, is a powerful oxidizing agent. Its chemical formula is HSO_5. This compound belongs to the family of peroxoacids, which are characterized by the presence of an extra oxygen atom compared to their corresponding acids, making them significantly more reactive.
Production Methods
The production of Caros Acid is a process of interest due to its application across various industries. The acid is typically produced through the reaction of hydrogen peroxide (H_2O_2) and concentrated sulfuric acid (H_2SO_4). The steps involved in the production are as follows:
- Mixing: Hydrogen peroxide is mixed with concentrated sulfuric acid.
- Cooling: The mixture is cooled to manage the exothermic reaction, which releases heat.
- Separation: Caros Acid, being less dense, is separated from the mixture.
This method is efficient and produces Caros Acid that is ready for industrial use.
Key Properties
Caros Acid possesses unique physical and chemical properties that make it highly valuable:
- Physical State: It is a colorless liquid at room temperature.
- Solubility: Highly soluble in water, which facilitates its use in aqueous solutions.
- Stability: It is relatively stable under cool, acidic conditions but decomposes in the presence of heat or neutral pH.
- Oxidizing Ability: Its most notable property is its strong oxidizing ability, capable of breaking down complex molecules.
Applications
Caros Acid finds applications in several industries, leveraging its oxidizing power:
- Pulp and Paper Industry: Used for bleaching wood pulp, improving paper whiteness.
- Waste Water Treatment: Breaks down pollutants and organic compounds.
- Mining: Helps in the extraction of metals by oxidizing the ores.
- Chemical Synthesis: Acts as an oxidizer in the synthesis of various chemicals.
Marshalls Acid Overview
Definition and Composition
Marshalls Acid, or Peroxydisulfuric acid, is known for its potent oxidizing capabilities, more so than Caros Acid. Its chemical formula is H_2S_2O_8. This compound is distinguished by its two sulfur atoms and an additional oxygen atom, which confer it a higher level of reactivity and stability compared to many other oxidizing agents.
Production Methods
Marshalls Acid is produced primarily through the electrolysis of concentrated sulfuric acid, which involves passing an electric current through the acid. The key steps in this process include:
- Electrolysis Setup: Concentrated sulfuric acid is placed in an electrolysis chamber.
- Current Application: An electric current is applied, causing the sulfuric acid to undergo electrolysis.
- Acid Formation: The process yields Peroxydisulfuric acid along with other by-products.
This production method is notable for its efficiency and the purity of the acid produced.
Key Properties
The properties of Marshalls Acid contribute to its wide range of applications:
- Physical State: It is a colorless solid or highly concentrated liquid.
- Solubility: Soluble in water, though less so than Caros Acid.
- Stability: More stable than Caros Acid, especially in solid form.
- Oxidizing Power: Has a higher oxidizing power, capable of initiating more challenging chemical reactions.
Applications
Marshalls Acid is utilized in various sectors, including:
- Manufacturing of Dyes and Pigments: Used in the synthesis of complex dyes.
- Chemical Industry: Serves as a catalyst in the polymerization process.
- Electronics: Employed in the cleaning and etching of semiconductor surfaces.
- Environmental Applications: Plays a role in advanced oxidation processes for water treatment.
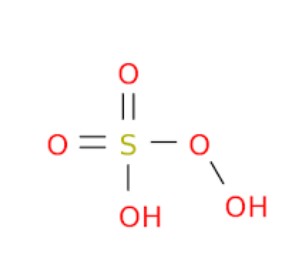
Comparative Analysis
Chemical Structure
Caros Acid (HSO_5) and Marshalls Acid (H_2S_2O_8) present fundamental differences in their chemical structures that account for their distinct properties and applications. Caros Acid features a single sulfur atom bonded to an additional oxygen atom, compared to sulfuric acid. This makes it a powerful oxidizing agent. Marshalls Acid, however, contains two sulfur atoms, each connected to two extra oxygen atoms, providing it with a stronger oxidizing capacity and greater stability. The additional oxygen atoms in Marshalls Acid account for its enhanced ability to participate in more vigorous and demanding oxidation reactions.
Production Differences
The production of Caros and Marshalls Acids involves distinct methods, reflecting their chemical composition differences. Caros Acid is typically produced by reacting hydrogen peroxide with sulfuric acid, a straightforward process that can be conducted at room temperature with careful control of reaction conditions to prevent excessive heat generation. In contrast, Marshalls Acid is produced through the electrolysis of sulfuric acid, a method that requires an electric current to induce the formation of the acid. This process is more energy-intensive and requires specialized equipment to handle the electrolysis safely.
Physical Properties
Caros Acid, being highly soluble in water, is usually found in liquid form and is known for its strong oxidizing properties. It has a lower stability compared to Marshalls Acid, especially at higher temperatures or when not stored properly. On the other hand, Marshalls Acid, also soluble in water, can exist as a colorless solid or a highly concentrated liquid. It exhibits greater stability, making it suitable for storage and use in a wider range of conditions without rapid decomposition.
Chemical Properties
The oxidizing power of Caros and Marshalls Acids is a key chemical property that distinguishes them. Caros Acid is a strong oxidizer, capable of breaking down various organic and inorganic compounds. However, Marshalls Acid’s ability to release more oxygen atoms during reactions makes it a superior oxidizing agent, enabling it to oxidize substances that Caros Acid cannot. Additionally, Marshalls Acid’s chemical stability under various conditions allows for its use in applications where a controlled, powerful oxidation is necessary.
Industrial Uses
Both acids find extensive use across industries, yet their applications are tailored to their unique properties. Caros Acid is often employed in pulp bleaching and water treatment applications, where its oxidizing capability is essential for breaking down pollutants and enhancing the quality of paper products. Marshalls Acid, with its higher oxidizing power, finds use in the manufacture of chemicals and dyes, where more intensive oxidation is required. It’s also utilized in the electronics industry for cleaning and etching processes, showcasing its versatility.
Safety and Environmental Concerns
Handling and Storage
Proper safety measures are paramount when handling both Caros and Marshalls Acids due to their corrosive nature and strong oxidizing properties. Protective gear, including gloves, goggles, and face shields, should be worn to prevent skin and eye contact. Furthermore, both acids should be stored in cool, well-ventilated areas away from organic materials or combustibles to prevent accidental reactions or decomposition, which could lead to the release of harmful gases.
Environmental Impact
The environmental implications of using Caros and Marshalls Acids are significant. While they can be beneficial in treating wastewater by breaking down pollutants, their disposal must be managed carefully to prevent environmental harm. Both acids can lead to acidification of water bodies if not neutralized properly before disposal. Additionally, their production and use must be carefully controlled to minimize the release of harmful by-products into the environment.
Future Prospects
Research and Development
Ongoing research into Caros and Marshalls Acids focuses on improving their production efficiency, stability, and environmental friendliness. Innovations in catalysts and reaction conditions are being explored to reduce the energy consumption and by-products of their production processes. Furthermore, new applications in organic synthesis, pollution control, and renewable energy storage are under investigation, expanding their utility in modern technology and industry.
Potential Applications
The potential future applications of Caros and Marshalls Acids are vast. Their strong oxidizing properties could play a crucial role in developing cleaner energy sources, such as in the production of hydrogen fuel through water electrolysis. Additionally, advancements in chemical synthesis may enable the use of these acids in the creation of novel materials with unique properties for use in nanotechnology, biotechnology, and advanced manufacturing processes.
Frequently Asked Questions
What is Caros Acid used for?
Caros Acid is primarily used as a powerful oxidizing agent in organic synthesis and pollution control. Its potent oxidizing properties make it suitable for applications such as pulp bleaching in the paper industry, wastewater treatment, and in the synthesis of various organic compounds where a strong oxidizer is required.
How is Marshalls Acid produced?
Marshalls Acid is produced through the electrolysis of sulfuric acid, where an electric current is passed through concentrated sulfuric acid, resulting in the formation of Peroxydisulfuric acid. This process requires specific conditions, including a controlled temperature and the use of a platinum electrode to facilitate the electrolysis.
Are Caros and Marshalls Acids dangerous?
Yes, both Caros and Marshalls Acids are highly corrosive and can pose significant risks if not handled properly. They require specific safety measures, including appropriate protective equipment and storage conditions, to prevent harm to individuals and the environment. Safety data sheets for each acid provide detailed information on handling, storage, and first aid measures.
What distinguishes Caros Acid from Marshalls Acid?
The key distinction between Caros and Marshalls Acids lies in their chemical structure and oxidizing power. Caros Acid (Peroxymonosulfuric acid) has a single sulfur atom bonded to three oxygen atoms, making it a strong but less stable oxidizer. Marshalls Acid (Peroxydisulfuric acid), with two sulfur atoms and an extra oxygen atom, offers greater stability and a higher oxidizing potential, making it suitable for more demanding applications.
Conclusion
Understanding the differences between Caros Acid and Marshalls Acid not only enriches our knowledge of chemistry but also highlights the intricacies involved in choosing the right chemical for specific industrial applications. These distinctions, grounded in their chemical properties, production methods, and applications, play a crucial role in their effectiveness and safety in various contexts.
As the chemical industry continues to evolve, the significance of these acids and their nuanced differences underscores the importance of targeted research and development. By delving deeper into their potential, the industry can harness these compounds more effectively, paving the way for innovations that prioritize efficiency, safety, and environmental sustainability.


