Organic compounds play a pivotal role in various industries, from pharmaceuticals to textiles, offering a vast array of applications that impact our daily lives. Among these, azo and diazo compounds stand out for their unique chemical properties and widespread use. Both groups of compounds are renowned for their vibrant colors and reactivity, which make them invaluable in several fields, including dye manufacturing and organic synthesis.
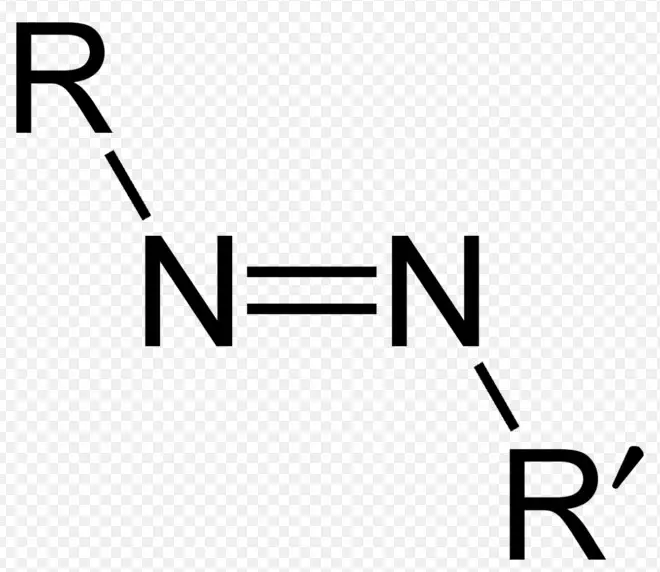
The difference between azo and diazo compounds lies in their chemical structure and applications. Azo compounds are characterized by the presence of a nitrogen-nitrogen double bond (N=N) connecting two aryl groups, making them essential in the production of dyes and pigments. In contrast, diazo compounds contain a diazo group (two nitrogen atoms connected by a double bond, with one of the nitrogens also bonded to a carbon atom), and are crucial in high-energy materials, photography, and as intermediates in organic synthesis.
Understanding the distinction between these compounds is crucial for chemists and industries that rely on these chemicals for producing a range of products, from colorful fabrics to intricate synthetic molecules. The unique properties of azo and diazo compounds, such as their reactivity and the conditions under which they are stable, highlight their significance in both research and commercial applications, underscoring the importance of detailed knowledge about these substances.

Azo Compounds
Overview
Azo compounds stand as a significant class of organic molecules recognized for their distinctive nitrogen-nitrogen double bond (N=N). This bond connects two aryl groups, forming a bridge that is central to the compound’s vibrant colors and wide-ranging applications. Azo compounds are primarily celebrated for their roles in creating dyes and pigments, showcasing an array of hues that span the spectrum.
Definition and Chemical Structure
An azo compound is defined by its azo group (-N=N-), where two nitrogen atoms are double-bonded, each linked to an aryl or alkyl group. This structure is not only pivotal for the compound’s coloration but also contributes to its chemical stability and reactivity. The versatility of the azo group allows it to participate in various chemical reactions, making azo compounds adaptable for numerous applications.
Types and Examples
Azo compounds can be broadly categorized based on their application and chemical structure. They include:
- Solvent Dyes: Used in organic solvents, oils, and as indicators.
- Acid Dyes: Applied to fibers such as silk, wool, and nylon.
- Direct Dyes: Employed for coloring cellulose fibers directly.
Notable examples include Methyl Red, an acid-base indicator, and Direct Blue 15, a common textile dye.
Properties
Physical Characteristics
Azo compounds are renowned for their vivid colors, ranging from yellow to deep red, and even to black. The specific color depends on the nature of the aryl groups attached to the azo group. These compounds tend to have moderate to high molecular weights and can show different states of matter, predominantly solids, at room temperature.
Chemical Behavior
The chemical reactivity of azo compounds is influenced by the azo bond and the substituents attached to the nitrogen atoms. They can undergo:
- Electrophilic Substitution Reactions: In aromatic azo compounds, the azo group influences the reactivity of the ring.
- Reduction: Azo compounds can be reduced to amines, which significantly alters their properties.
Applications
Dyes and Pigments
Azo compounds are extensively used in the dye industry. Their ability to offer a wide palette of colors makes them ideal for:
- Textiles: Fabric coloring to achieve vibrant and long-lasting shades.
- Leather: Used in tanning and dyeing processes.
Polymer Industry
In polymers, azo compounds contribute to:
- Coloring Plastics: Ensuring consistent and bright colors in plastic materials.
- Thermal Stability: Some azo compounds improve the thermal stability of polymers.
Analytical Chemistry
Azo compounds serve as pH indicators and analytical reagents due to their color-changing properties under different conditions, facilitating the detection and quantification of various substances.
Diazo Compounds
Overview
Diazo compounds share a foundational feature with azo compounds—the presence of nitrogen atoms. However, diazo compounds are defined by their two nitrogen atoms (N2+), with one typically connected to a carbon atom, forming a distinctive diazo group. This configuration lends itself to high reactivity and the ability to form complex structures, marking its utility in several scientific and industrial fields.
Definition and Formation
A diazo compound typically consists of a diazonium group (R-N2+), where R represents an aryl or alkyl group. The formation of diazo compounds is primarily achieved through the diazotization reaction, which involves treating a primary aromatic amine with nitrous acid under cold conditions.
Types and Examples
Diazo compounds are diverse, with applications ranging from synthesis intermediates to imaging agents. Examples include:
- Diazo Dyes: Used for textile and paper coloring.
- Diazo Resins: Employed in coatings and adhesives for their strong binding properties.
Properties
Stability and Reactivity
Diazo compounds are generally less stable than azo compounds, with their stability heavily dependent on the nature of the substituents. Their reactivity allows for:
- Cycloaddition Reactions: Forming rings and complex structures.
- Ylide Formation: Engaging in reactions pivotal for organic synthesis.
Light Sensitivity
One of the hallmark properties of diazo compounds is their sensitivity to light, a trait that is leveraged in:
- Photolithography: For creating microscale patterns.
- Photoresists: In the manufacture of electronic components.
Applications
Photography and Printing
In photography and printing, diazo compounds’ light sensitivity is exploited for the development of images on paper and films. They are key in:
- Blueprints: Producing copies of architectural and engineering designs.
- Photographic Papers: Where exposure to light induces color changes.
Synthetic Intermediates
Diazo compounds are invaluable in synthetic chemistry, acting as intermediates in the synthesis of complex molecules, including:
- Pharmaceuticals: Creating active pharmaceutical ingredients (APIs).
- Agrochemicals: Formulating pesticides and herbicides.
Biological Research
In biological research, diazo compounds are used in:
- Molecular Labeling: Attaching to biomolecules without disrupting their function.
- Protein Engineering: As tools for modifying proteins and peptides.
Comparative Analysis
Chemical Structure
Bonds and Functional Groups
Azo and diazo compounds are characterized by their nitrogen-containing bonds, but the specifics of their structures set them apart significantly. Azo compounds have a nitrogen-nitrogen double bond (N=N), connecting two aryl or alkyl groups, which is the source of their vibrant colors and versatility in applications. Diazo compounds, on the other hand, feature a diazonium group (R-N2+), where a carbon is bonded to two nitrogen atoms, one of which carries a positive charge. This structure contributes to the high reactivity and less stability of diazo compounds compared to their azo counterparts.
Reactivity
Conditions and Outcomes
The reactivity of these compounds is a tale of two chemistries. Azo compounds are generally stable under normal conditions and can undergo specific reactions such as azo coupling and reduction to form amines. Diazo compounds, due to their diazonium group, are much more reactive, particularly under light or heat, which can lead to the generation of carbenes or insertion into other molecules.
Differences in Applications
The difference in reactivity between azo and diazo compounds translates into a wide variety of applications. Azo compounds are predominantly used in dyeing textiles and making colorants due to their stability and vibrant colors. Diazo compounds, given their reactivity, are crucial in synthetic chemistry for the creation of complex molecules and are also used in photographic materials due to their light sensitivity.
Safety and Environmental Impact
Toxicity and Hazards
The safety aspect of azo and diazo compounds cannot be overstated. Some azo dyes can decompose to form aromatic amines, some of which are known to be carcinogenic. This has led to regulations on their use, especially in clothing and food products. Diazo compounds, given their high reactivity, can pose risks of explosiveness under certain conditions, and their handling requires strict safety measures.
Disposal and Regulations
The disposal of azo and diazo compounds must be managed with care to minimize environmental impact. Regulations often dictate the need for treatment processes to break down these compounds into less harmful substances before disposal. The industry continues to seek greener alternatives and safer handling practices to mitigate the risks associated with these compounds.
Technological Advancements
Innovations in Azo Compounds
Enhanced Stability and Eco-friendliness
Recent innovations have focused on making azo compounds more environmentally friendly and stable. This includes the development of azo dyes that do not release harmful amines upon decomposition and the use of bio-based materials for dye production. Efforts are also being made to improve the lightfastness and washfastness of azo dyes, making them more durable in various applications.
Breakthroughs in Diazo Chemistry
Novel Applications and Synthesis Methods
Diazo compounds have seen significant advancements in their synthesis and application. One area of progress is the development of safer, more efficient synthesis methods that reduce the risks associated with diazo compound preparation. Additionally, novel applications, such as their use in 3D printing technologies and as precursors for making complex molecular structures in pharmaceuticals, highlight the versatility and importance of diazo chemistry in modern science.
Frequently Asked Questions
What Are Azo Compounds?
Azo compounds are organic compounds characterized by the presence of a nitrogen-nitrogen double bond (N=N) linking two aryl groups. These compounds are predominantly used in the manufacture of dyes and pigments due to their vivid colors and ability to bind to fabrics and other materials, providing long-lasting colorfastness. Their versatility and stability under various conditions make them indispensable in the dyeing industry.
How Are Diazo Compounds Used in Photography?
Diazo compounds find extensive applications in photography, particularly in the production of light-sensitive materials. When exposed to light, these compounds undergo decomposition, leading to a chemical reaction that produces a dye. This property is exploited in creating detailed images on paper or film, making diazo compounds essential for traditional photographic processes and the printing industry.
What Makes Diazo Compounds Unique in Organic Synthesis?
Diazo compounds are unique in organic synthesis due to their high reactivity and versatility as intermediates. They can undergo a variety of reactions, including cycloadditions, insertions, and substitutions, allowing chemists to construct complex molecules with high precision. Their ability to generate carbenes, which can insert into C-H and other bonds, makes them invaluable tools for building molecular structures in pharmaceuticals and synthetic organic chemistry.
Are Azo Compounds Safe?
The safety of azo compounds depends on their specific structure and application. While many azo dyes are safe and widely used in textiles and food colorings, certain azo compounds can release aromatic amines upon reduction, which may be carcinogenic. Consequently, the use of some azo dyes is regulated or banned in certain applications, underscoring the importance of understanding and managing the risks associated with their use.
Conclusion
The distinctions between azo and diazo compounds underscore the diversity and complexity of organic chemistry, revealing the depth of thought and investigation that goes into utilizing these substances in various applications. Their unique properties and applications not only highlight the ingenuity of chemical synthesis but also its practical implications in everyday products and advanced technologies.
As we continue to explore the nuances of these compounds, their role in advancing both scientific understanding and industrial applications cannot be overstated. The ongoing research and development in this area promise to uncover new uses and methods of production that will further enhance the value of azo and diazo compounds, solidifying their place in the annals of chemical achievement.