Organic solvents are pivotal in the realm of chemical synthesis and industrial applications, among which anisole and diethyl ether stand out due to their unique properties and uses. These compounds are widely utilized across various sectors, each serving specific roles that hinge on their chemical nature and behavior. While both are essential in scientific and manufacturing processes, understanding their differences is crucial for appropriate application and safety.
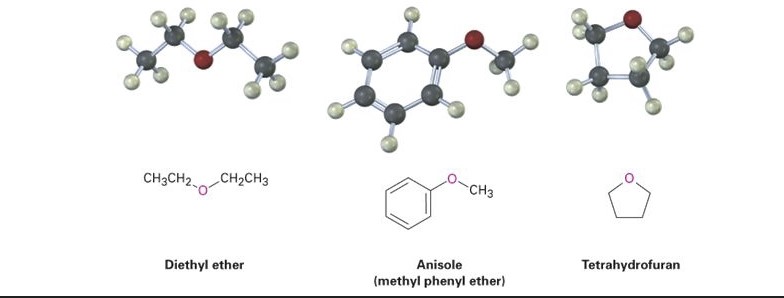
Anisole and diethyl ether are two distinctly different organic solvents; anisole is an aromatic ether, whereas diethyl ether is a simple ether. Anisole has a methyl group attached to an oxygen bonded to a benzene ring, contributing to its distinct aromatic properties and higher boiling point compared to diethyl ether, which consists of two ethyl groups linked by an oxygen atom, making it highly volatile and flammable.
In industrial and scientific contexts, these solvents are chosen based on their physical and chemical properties. Anisole is favored in organic synthesis and the perfume industry for its stability and aromatic character, while diethyl ether is predominantly used as a general anesthetic, in laboratories as a solvent, and as a starting fluid for engines due to its volatility and quick evaporation.
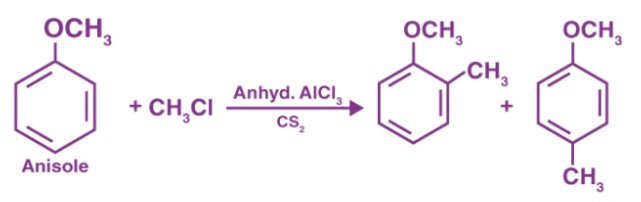
Anisole Overview
Definition and Chemical Structure
Anisole, also known as methoxybenzene, is an organic compound with the formula C₆H₅OCH₃. This chemical structure consists of a benzene ring substituted with a methoxy group (-OCH₃). It is a derivative of phenol, with the hydrogen atom of the hydroxyl group replaced by a methyl group attached through an oxygen atom.
Physical Properties
Anisole is characterized by its clear, colorless appearance and a pleasant, sweet smell reminiscent of anise seed. It has a boiling point of 154°C, which is significantly higher than many simple ethers due to the presence of the aromatic ring. Anisole is also slightly soluble in water but mixes well with most organic solvents, making it versatile in various chemical applications.
Common Uses and Applications
Anisole is extensively used in the chemical industry, especially in:
- Synthesis of dyes and pharmaceuticals: It serves as a precursor to more complex chemical compounds.
- Perfumery: Its sweet, anise-like fragrance is valued for creating aromatic profiles in perfumes and scents.
- Organic synthesis: Anisole reacts in many types of chemical reactions, serving as a protecting group for phenols.
Diethyl Ether Overview
Definition and Chemical Structure
Diethyl ether, commonly known as ether, is a simple ether with the formula (C₂H₅)₂O. It consists of two ethyl groups connected by an oxygen atom. This structure is responsible for its properties as a highly volatile, flammable liquid.
Physical Properties
Diethyl ether is a colorless, volatile liquid with a distinctive, pungent odor. It has a low boiling point of about 34.6°C, which contributes to its rapid evaporation at room temperature and its use as an anesthetic due to this property. It is also highly flammable, making its handling and storage critical.
Common Uses and Applications
Diethyl ether’s applications are diverse and include:
- General anesthetic: Historically used in surgery for its anesthetic properties.
- Laboratory solvent: Widely used in scientific labs for extractions and as a reaction medium.
- Starting fluid: For engines, especially in colder conditions, due to its ability to vaporize quickly.
Chemical Properties
Reactivity and Stability
Anisole and diethyl ether exhibit different reactivities due to their structures. Anisole, with its aromatic ring, is relatively stable under most conditions but can participate in electrophilic substitution reactions. Diethyl ether, however, can form peroxides on exposure to air, making it less stable and more dangerous over time.
Comparison of Boiling Points
The boiling point of anisole is higher than that of diethyl ether, due to the aromatic ring in anisole which provides greater thermal stability and a higher molecular weight. This difference affects their uses in industrial and laboratory settings, with diethyl ether being preferable for processes requiring lower temperatures.
Solubility Characteristics
Anisole’s solubility in water is limited, but it dissolves well in organic solvents. Diethyl ether is similar but even less soluble in water, reflecting its nonpolar nature. Both ethers can act as good solvents for organic reactions due to these properties.
Production Methods
Synthesis of Anisole
Anisole is typically produced by the Williamson ether synthesis, involving the reaction of sodium phenoxide (from phenol) with methyl chloride or dimethyl sulfate. This method is favored for its simplicity and efficiency.
Synthesis of Diethyl Ether
Production of diethyl ether is generally achieved through the acid-catalyzed dehydration of ethanol. This reaction takes place under reflux with a strong acid like sulfuric acid, which helps in removing water formed during the synthesis.
Industrial Scale Production Differences
While both anisole and diethyl ether can be synthesized through relatively straightforward chemical reactions, the scale and safety measures differ significantly. Diethyl ether’s synthesis requires rigorous safety protocols due to its high volatility and flammability. Anisole’s production, while also needing careful handling due to its toxic nature, is generally less hazardous.
Health and Safety
Toxicity Levels
Both anisole and diethyl ether pose health risks. Anisole is toxic if ingested or inhaled in large amounts, and diethyl ether is both toxic and highly flammable, with risks of respiratory irritation and neurological effects from inhalation.
Handling and Storage Requirements
Safe handling of these chemicals involves:
- Proper ventilation: To prevent vapor accumulation.
- Cool and dry storage: Especially for diethyl ether to prevent peroxide formation.
- Avoiding ignition sources: Due to the flammability of diethyl ether.
Common Hazards and Precautions
- Anisole requires precautions to avoid skin and respiratory contact.
- Diethyl ether’s storage containers should be dated and checked regularly for peroxides if stored for long periods.
- Safety equipment such as fume hoods, gloves, and fire extinguishers must be readily available when handling these solvents.
Environmental Impact
Biodegradability and Persistence
The environmental footprint of chemicals like anisole and diethyl ether includes their biodegradability and persistence. Anisole, with its aromatic structure, is moderately biodegradable under aerobic conditions. However, it can persist in the environment under anaerobic conditions, such as in sediments or groundwater, where less oxygen is available. In contrast, diethyl ether is more volatile, leading to rapid evaporation and less persistence in soil and water. However, its presence in the air can contribute to photochemical smog under certain conditions.
Impact on Air and Water Quality
Both anisole and diethyl ether can impact air and water quality if not managed properly:
- Anisole can release volatile organic compounds (VOCs) into the air during its use in industrial applications, contributing to air pollution and potential health hazards.
- Diethyl ether’s high volatility makes it a significant contributor to air quality issues, especially in enclosed spaces where its vapors may concentrate.
- In water, anisole is moderately soluble, potentially affecting aquatic life if discharged in significant quantities. Diethyl ether’s solubility in water is low, but its toxicity to aquatic organisms is a concern.
Safety Measures and Regulations
To mitigate environmental impacts, various safety measures and regulations are enforced:
- Emission controls and ventilation systems in factories to reduce VOC emissions.
- Waste management practices to ensure that chemical residues do not enter the water supply.
- Strict adherence to guidelines set by environmental agencies, like the EPA in the United States, for the handling and disposal of chemical substances.
Applications in Industry
Use in Pharmaceutical Synthesis
Anisole and diethyl ether are pivotal in pharmaceutical synthesis due to their chemical reactivity and solvent properties:
- Anisole is used to synthesize active pharmaceutical ingredients (APIs) that require complex aromatic structures.
- Diethyl ether serves as a solvent and a reaction medium in the synthesis of various drugs, helping to control reaction environments and extract final products.
Role in Perfumery and Flavoring
Anisole is particularly valued in the perfumery and flavoring industries for its pleasant, sweet-smelling characteristic reminiscent of anise:
- It is used as a base note in perfumes, providing depth and longevity to fragrance compositions.
- In flavoring, it imparts a sweet, aromatic taste to food products, enhancing their overall sensory quality.
Diethyl ether, due to its strong odor, is not used in perfumery or flavoring.
Applications in Research and Development
Both anisole and diethyl ether are used extensively in research and development:
- Anisole in research related to organic chemistry and materials science, particularly in the study of phenolic compounds and their derivatives.
- Diethyl ether is commonly used in laboratories for organic extractions, as a solvent in chromatography, and in various other experimental procedures.
Economic Aspects
Market Trends and Demands
The demand for anisole and diethyl ether varies based on their applications and industrial needs. The market for anisole is driven by its use in high-value sectors like pharmaceuticals and perfumery. The market for diethyl ether has been influenced by its traditional uses in laboratories and as an anesthetic, though its use in the latter has decreased due to the availability of safer alternatives.
Cost Implications for Production
The cost of producing anisole and diethyl ether can be influenced by several factors:
- Raw material availability: Prices of ethanol and methanol, key precursors for diethyl ether and anisole respectively, significantly affect production costs.
- Regulatory compliance: Meeting environmental and safety regulations can increase operational costs.
- Technological advancements: Innovations in production technology may reduce costs over time.
Future Outlook in Industrial Applications
The future of anisole and diethyl ether in industrial applications appears robust but will likely evolve with technological and environmental considerations. For anisole, expanding applications in high-performance polymers and electronics are anticipated. Diethyl ether’s role may shrink in medical applications but continue to be strong in chemical research and synthesis, driven by its effectiveness as a solvent and in extractions.
Frequently Asked Questions
What is Anisole Used For?
Anisole is primarily used in the synthesis of other chemicals, particularly in the pharmaceutical and perfume industries. Its ability to enhance certain chemical reactions makes it valuable for producing more complex organic compounds.
How is Diethyl Ether Produced?
Diethyl Ether is typically produced through the acid ether synthesis, where ethanol is reacted with sulfuric acid. This method has been historically significant in both laboratory and industrial scales.
What are the Safety Concerns with Diethyl Ether?
Diethyl ether is highly flammable and volatile, leading to specific storage and handling precautions. It requires cool, ventilated environments away from sparks or open flames to prevent fire hazards.
Is Anisole Environmentally Safe?
While anisole is not as volatile as diethyl ether, it poses its own environmental risks. It is moderately toxic and requires careful disposal to prevent water and soil contamination.
Can Diethyl Ether be Used in Perfumery?
Diethyl ether is not typically used in perfumery due to its strong, pungent smell and high volatility. It evaporates too quickly and can overpower other delicate fragrance notes.
Conclusion
Exploring the differences between anisole and diethyl ether reveals a fascinating glimpse into how chemical structure influences physical properties and application suitability. Each solvent’s utility and handling requirements reflect its unique characteristics, emphasizing the importance of choosing the right solvent for specific industrial or research needs.
As advancements in chemical synthesis and environmental safety continue to evolve, the roles of anisole and diethyl ether may expand or shift, guided by ongoing research and technological development. Understanding their distinct qualities not only enhances safety and efficiency in their use but also underpins innovative applications in future scientific endeavors.

