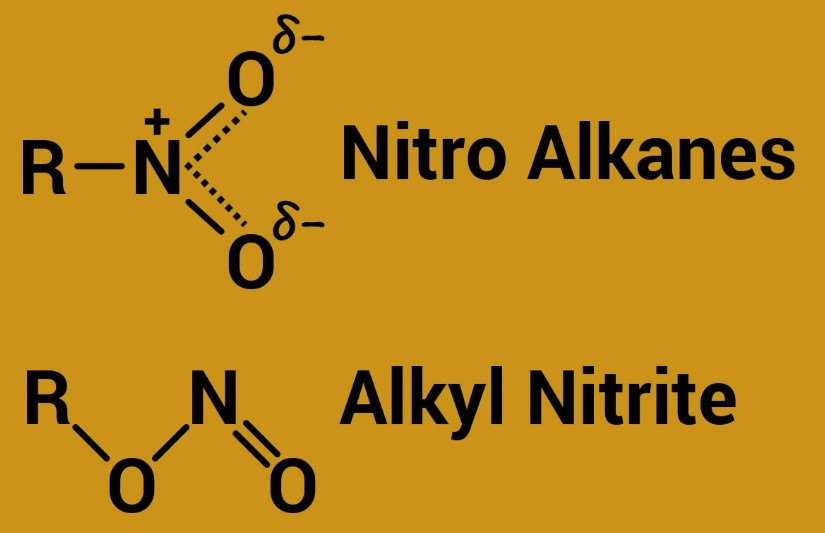
In the realm of organic chemistry, the distinction between compounds often goes beyond mere nomenclature to encompass differences in structure, reactivity, and application. Two such compounds that frequently emerge in discussions due to their widespread use and significant impact are Alkyl Nitrite and Nitro Alkane. Each holds a unique position in both industrial processes and pharmaceutical applications, underscoring the importance of grasping their distinct characteristics.
Alkyl Nitrites are organic compounds characterized by a nitrite group (–ONO) attached to an alkyl chain, known for their role in medicine and as recreational substances. Nitro Alkanes, on the other hand, feature a nitro group (–NO2) bonded to an alkane, serving critical functions in the synthesis of various chemicals and pharmaceuticals. Despite their seemingly similar names, these compounds diverge significantly in terms of chemical structure, reactivity, and usage.
The differences between Alkyl Nitrite and Nitro Alkane extend into their applications, health implications, and environmental impact. Alkyl Nitrites are primarily employed in medical settings and as additives, whereas Nitro Alkanes are pivotal in the production of explosives, solvents, and several organic syntheses. This contrast underlines the necessity for chemical knowledge in navigating their safe and effective use, along with understanding the potential health risks and environmental considerations associated with each.
Alkyl Nitrite Basics
Definition and Structure
Alkyl Nitrite is an organic compound characterized by the formula R-ONO, where ‘R’ represents an alkyl group. The structure consists of an alkyl chain attached to a nitrite functional group. This setup imparts unique chemical properties to alkyl nitrites, distinguishing them from other organic compounds.
Common Uses and Applications
Alkyl nitrites have a variety of applications across different fields:
- Medical: Used as vasodilators to treat heart conditions like angina.
- Recreational: Known as “poppers,” they provide a brief euphoria and muscle relaxation.
- Industrial: Employed in synthesis reactions and as solvents due to their reactivity.
Health and Safety Considerations
Despite their utility, alkyl nitrites pose health risks. Exposure can lead to:
- Lowered blood pressure
- Dizziness and headaches
- Potential risk of dependency in recreational use
Safety measures include proper ventilation, use of protective gear, and adherence to handling guidelines.
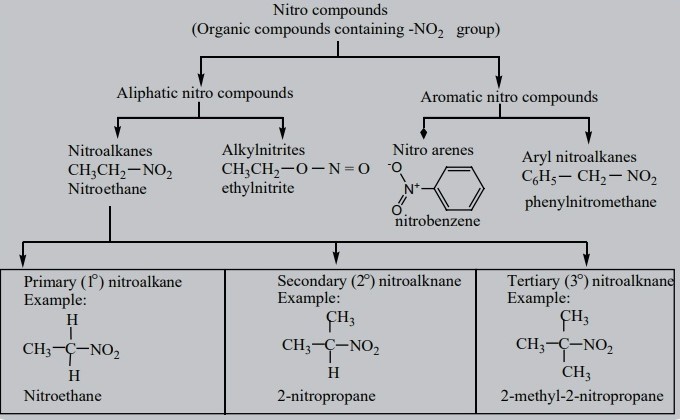
Nitro Alkane Essentials
Definition and Molecular Structure
Nitro Alkane is another class of organic compounds, defined by the presence of one or more nitro groups (–NO2) attached to an alkane. This molecular structure confers distinct properties and reactivity patterns, making nitro alkanes crucial in various applications.
Industrial and Pharmaceutical Applications
Nitro alkanes find applications in:
- Explosives: Their high reactivity makes them suitable for explosive materials.
- Pharmaceuticals: Used as intermediates in the synthesis of drugs.
- Solvents: Due to their chemical stability, they’re used as solvents in industrial processes.
Safety and Environmental Impact
Handling nitro alkanes requires caution due to:
- Toxicity: They can be harmful if ingested or inhaled.
- Environmental concerns: Improper disposal can lead to water and soil contamination.
Key Differences
Chemical Structure Contrast
The primary difference lies in the functional group: Alkyl Nitrites have a nitrite group (–ONO), whereas Nitro Alkanes contain a nitro group (–NO2). This distinction significantly affects their chemical behavior and applications.
Reactivity and Stability
Alkyl Nitrites are more reactive towards nucleophilic agents, while Nitro Alkanes exhibit stability but can act as powerful electrophiles in organic reactions.
Usage in Industry and Medicine
While alkyl nitrites mainly find use in medicine and as recreational substances, nitro alkanes are pivotal in industrial manufacturing and the pharmaceutical industry.
Health and Environmental Concerns
Both classes of compounds require careful handling due to their health and environmental impacts, emphasizing the need for safety protocols and regulatory compliance.
Chemical Structure and Properties
Alkyl Nitrite Structure
The molecular composition of alkyl nitrites allows for quick interaction with biological systems, leading to their vasodilatory effects. Their physical properties include volatility and a characteristic fruity odor.
Nitro Alkane Structure
Nitro Alkanes are characterized by their stability and relatively low reactivity under normal conditions. Their physical properties vary significantly across different compounds, influencing their solubility and boiling points.
Reactivity and Stability
Alkyl Nitrite Reactivity
Alkyl nitrites are known for their rapid reactions with other compounds, a feature that underpins their medical and recreational use. Their stability is influenced by temperature, concentration, and the presence of other chemicals.
Nitro Alkane Reactivity
Compared to alkyl nitrites, nitro alkanes have a different reactivity profile, capable of undergoing various chemical transformations, including reductions and nucleophilic substitutions. Their stability under various conditions makes them valuable in synthetic chemistry.
Applications in Industry
Alkyl Nitrite Uses
Role in Manufacturing Processes
Alkyl Nitrites serve as pivotal agents in synthetic organic chemistry. Their ability to act as solvents and reagents in various chemical reactions enhances the efficiency of synthesizing complex molecules. This utility is particularly evident in the production of paints, polymers, and pharmaceuticals, where their properties facilitate smoother reactions and product formulation.
Application in Medical Fields
In the medical realm, Alkyl Nitrites are renowned for their vasodilatory effects, making them essential in treating conditions like heart diseases and angina. They work by relaxing blood vessels, thereby improving blood flow and oxygen delivery to cardiac tissues. This therapeutic application underscores the compound’s significance beyond its recreational use, highlighting its potential in emergency medicine and chronic condition management.
Nitro Alkane Uses
Industrial Applications
Nitro Alkanes find extensive application in the manufacturing sector, especially in the creation of explosives, synthetic fibers, and plastics. Their chemical reactivity and stability make them ideal for processes requiring high-energy compounds. Moreover, their role as intermediates in organic synthesis is invaluable, contributing to the development of a wide range of industrial chemicals.
Significance in Pharmaceuticals
The pharmaceutical industry benefits significantly from the unique properties of Nitro Alkanes. These compounds are crucial in synthesizing active pharmaceutical ingredients (APIs), serving as building blocks for drugs that treat various ailments. Their versatility and reactivity enable the design of complex drug molecules, facilitating advancements in medicinal chemistry and drug development.
Health Implications
Alkyl Nitrite Safety
Potential Health Risks
While Alkyl Nitrites offer therapeutic benefits, their misuse can lead to serious health risks, including respiratory issues, blood pressure fluctuations, and neurological effects. The rapid onset of their effects poses additional risks of accidental overexposure, especially in non-medical settings.
Safe Handling Practices
To mitigate health risks, strict handling practices are essential:
- Use in well-ventilated areas to prevent inhalation risks.
- Wear protective gear (gloves, goggles) during industrial handling.
- Educate users on the potential effects and proper use in recreational settings.
Nitro Alkane Concerns
Health Hazards
Exposure to Nitro Alkanes can lead to toxicological effects, including irritation of the skin, eyes, and respiratory system. Chronic exposure may result in more severe health outcomes, such as organ damage or cancer, highlighting the need for stringent occupational safety measures.
Environmental Impact and Safety Measures
The environmental persistence of Nitro Alkanes necessitates effective safety measures:
- Implement spill containment and disposal protocols to prevent environmental contamination.
- Monitor air and water quality in areas of high use to safeguard against long-term ecological damage.
- Employ alternative materials where possible to reduce reliance on hazardous chemicals.
Environmental Impact
Alkyl Nitrite and Ecology
Biodegradability and Toxicity
Alkyl Nitrites exhibit variable biodegradability, with some compounds posing risks to aquatic ecosystems due to their toxicity. The environmental impact largely depends on their concentration and exposure duration, underscoring the importance of regulated use and effective wastewater treatment practices.
Nitro Alkane Environmental Concerns
Pollution and Long-term Effects
Nitro Alkanes contribute to environmental pollution concerns, particularly regarding water and soil contamination. Their stability and potential to bioaccumulate necessitate careful management to prevent adverse effects on wildlife and ecosystems. Ongoing research into their degradability and toxicity aims to better understand and mitigate these long-term environmental impacts.
Frequently Asked Questions
What are Alkyl Nitrites used for?
Alkyl Nitrites, often recognized under names like “poppers,” have both recreational and medical uses. Medically, they serve as vasodilators to treat angina and heart conditions by relaxing blood vessels. Recreationally, they are used for their psychoactive effects, inducing a brief euphoria and muscle relaxation. Despite their popularity, it’s crucial to be aware of the potential risks and legal status in different jurisdictions.
How do Nitro Alkanes differ from Alkyl Nitrites?
The fundamental difference lies in their chemical structure and resultant properties. Nitro Alkanes contain a nitro group attached to an alkane chain, making them potent for use in explosives and as precursors in pharmaceuticals. Alkyl Nitrites, with their nitrite group, are known for their quick-acting vasodilatory effects. This structural variance accounts for their distinct reactivity, applications, and health impacts.
Are there health risks associated with Alkyl Nitrites and Nitro Alkanes?
Yes, both compounds pose health risks, albeit differing in nature due to their distinct chemical properties. Alkyl Nitrites can cause sudden drops in blood pressure, dizziness, and headaches, especially when misused. Nitro Alkanes, used industrially, are hazardous due to their potential toxicity and flammability, necessitating strict handling and exposure controls to mitigate risks of poisoning and environmental damage.
Conclusion
Understanding the nuanced differences between Alkyl Nitrite and Nitro Alkane is more than an academic exercise; it is a necessity for those involved in their production, application, and regulation. These compounds, integral to various sectors, demand respect for their properties, uses, and the safety measures required to handle them. The chemical structure dictates their reactivity and applications, emphasizing the importance of precise knowledge in leveraging their benefits while mitigating associated risks.
Reflecting on their environmental and health impacts further solidifies the need for informed usage and disposal practices. As the demand for these substances continues in various industries and medical fields, embracing a detailed understanding ensures both advancement and safety in their application. This exploration not only enriches our chemical knowledge but also guides responsible stewardship of these powerful compounds.