Acids play a pivotal role in the vast landscape of chemistry, each with its unique characteristics and applications. Among the myriad of acids, Adipic Acid and Salicylic Acid stand out due to their significant uses in various industries and healthcare. While they share the basic nature of being acids, their properties, applications, and effects on the environment and health differ markedly, making them subjects of interest for both scientists and the general public.
Adipic Acid and Salicylic Acid are two chemically distinct compounds with different molecular structures, industrial uses, and health implications. Adipic Acid is primarily used in the manufacturing of nylon and polyurethane, while Salicylic Acid is renowned for its role in skincare and medicine, particularly in treating acne and psoriasis. Their environmental impacts also vary, with Adipic Acid being a concern for its greenhouse gas emissions during production, and Salicylic Acid being considered more eco-friendly due to its biodegradability.
These acids’ relevance extends beyond their primary uses, touching upon areas like environmental sustainability, health safety, and technological advancements. The distinction between Adipic and Salicylic Acids underscores the diversity within chemical compounds and their roles in modern society. Understanding their differences not only enriches our scientific knowledge but also guides responsible industrial practices and informed personal health decisions.
Adipic Acid Basics
Definition and Chemical Structure
Adipic Acid, scientifically known as hexanedioic acid, is a white crystalline substance that is crucial in the production of nylon, which is used globally. Chemically, it consists of a six-carbon chain, terminated at each end by a carboxyl group (-COOH). This structure is pivotal to its functionality and applications in various industrial processes.
Industrial Applications
Adipic Acid boasts a wide array of industrial applications, primarily due to its role as a monomer in the production of nylon 6,6, a type of nylon that offers superior strength and thermal resistance. Additionally, it is used in:
- Polyurethane foams: These are prevalent in automotive and furniture industries for cushioning.
- Plasticizers: To increase plastic flexibility.
- Food industry: As a flavorant and acidity regulator.
The versatility of Adipic Acid in these applications highlights its importance in modern manufacturing processes.
Environmental Impact
The environmental impact of Adipic Acid production is a significant concern. The process emits nitrous oxide (N2O), a greenhouse gas approximately 300 times more potent than carbon dioxide. Reducing these emissions is critical in combating climate change, prompting the industry to seek greener alternatives and more efficient production methods.
Salicylic Acid Fundamentals
Definition and Chemical Structure
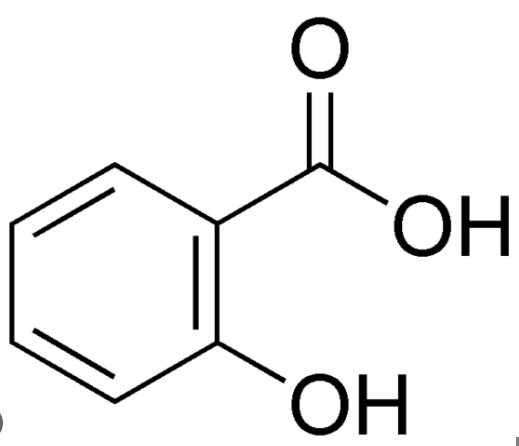
Salicylic Acid is a beta hydroxy acid (BHA) with profound implications in healthcare and cosmetics. Its molecular formula is C7H6O3, featuring a benzene ring bonded to a hydroxyl (-OH) and a carboxyl (-COOH) group. This unique structure enables it to penetrate oily substances and makes it effective in treating skin conditions.
Uses in Healthcare and Cosmetics
Salicylic Acid’s ability to exfoliate the skin by dissolving dead skin cells has made it a cornerstone in acne treatment products and skincare routines. It is found in:
- Acne creams and face washes: For its anti-inflammatory properties.
- Wart removers: Due to its keratolytic effects.
- Shampoos: To treat dandruff by exfoliating the scalp.
Its widespread use underscores its safety and effectiveness in managing skin health.
Natural Occurrence and Synthesis
Originally derived from willow bark, Salicylic Acid can now be synthesized through several chemical processes, including the Kolbe-Schmitt reaction. This synthesis allows for its mass production, ensuring its availability for various applications.
Key Differences
Chemical Properties
Adipic Acid and Salicylic Acid differ significantly in their chemical properties. Adipic Acid is aliphatic, lacking a benzene ring, which makes it less aromatic than Salicylic Acid. This distinction affects their solubility, reactivity, and the types of reactions they undergo, influencing their applications.
Industrial Uses Contrast
The primary use of Adipic Acid in nylon production contrasts with Salicylic Acid’s use in skincare. This difference in applications is rooted in their distinct chemical properties, showcasing how chemical structure dictates utility in industry.
Health and Environmental Implications
While Adipic Acid poses environmental challenges due to N2O emissions, Salicylic Acid’s impact is more related to health, particularly skin health. The environmental footprint of Salicylic Acid is comparatively lower, given its biodegradability and natural occurrence.
Chemical Structures Explained
Adipic Acid Structure Details
The structure of Adipic Acid, with its straight-chain dicarboxylic acid configuration, is essential for its polymerization reaction with hexamethylenediamine to produce nylon 6,6. Its molecular structure allows for the creation of strong, durable polymers.
Salicylic Acid Structure Insights
The aromatic nature of Salicylic Acid, due to its benzene ring, makes it highly effective in penetrating oily surfaces, a key attribute for its use in treating oily skin and acne. Its structure is also responsible for its anti-inflammatory properties.
Comparative Analysis
Comparing Adipic Acid and Salicylic Acid, we find fundamental differences in their molecular structures—Adipic Acid’s linear form versus Salicylic Acid’s aromatic ring. These structural differences directly influence their physical properties, applications, and environmental impacts. While both acids play vital roles in their respective domains, their distinct chemical structures cater to vastly different uses, from industrial manufacturing to personal skincare, illustrating the diverse potential of chemical compounds in improving various aspects of modern life.
Applications in Industry
Adipic Acid in Polymer Production
Adipic acid plays a crucial role in the polymer industry, particularly in the production of nylon 6,6. This process involves the polymerization of adipic acid with hexamethylenediamine, resulting in a strong and durable material widely used in textiles, automotive components, and various consumer goods. The characteristics of nylon 6,6, such as its high melting point and resistance to wear and tear, make it an ideal choice for a broad range of applications, from clothing to tire reinforcement.
Salicylic Acid in Skincare Products
Salicylic acid is a key ingredient in skincare due to its ability to penetrate the skin and exfoliate from within. This makes it highly effective in acne treatment products, such as cleansers, toners, and spot treatments. Its ability to reduce inflammation and unclog pores has cemented its status as a go-to solution for those battling acne and looking for clearer skin. Moreover, its use extends to anti-aging products, thanks to its capacity to promote cell renewal.
Diverse Uses in Other Sectors
Beyond their primary applications, both adipic acid and salicylic acid find roles in various sectors:
- Food Industry: Adipic acid serves as a food additive, enhancing flavor and regulating acidity.
- Medicinal Applications: Salicylic acid is used in wart removal products and aspirin, showcasing its versatility beyond skincare.
- Consumer Goods: From shoe soles made with adipic acid-based polymers to salicylic acid-infused hair care products, their impact is widespread.
Health and Safety
Adipic Acid Exposure Risks
Exposure to adipic acid, especially in industrial settings, can pose health risks. Inhalation of its dust or fumes can irritate the respiratory system, while skin or eye contact may cause irritation. Workplace safety measures, including proper ventilation and protective gear, are vital to minimize these risks.
Benefits and Risks of Salicylic Acid
Salicylic acid’s benefits for skin health are well-documented, particularly in treating acne and promoting smoother skin. However, its use can also lead to side effects such as skin irritation, dryness, or allergic reactions in some individuals. It’s important for users to start with lower concentrations and monitor their skin’s response, adjusting usage as needed.
Regulatory Guidelines
Both adipic acid and salicylic acid are subject to regulatory guidelines to ensure their safe use:
- Adipic Acid: Regulations focus on occupational exposure limits to protect workers in industrial environments.
- Salicylic Acid: In skincare and medicinal products, its concentration is regulated to prevent overexposure and potential side effects.
Environmental Impact
Production Process and Emissions for Adipic Acid
The production of adipic acid is energy-intensive and contributes to greenhouse gas emissions, notably nitrous oxide (N2O), a potent greenhouse gas. Efforts to reduce these emissions include technological advancements in production processes and the exploration of bio-based alternatives to traditional petrochemical sources.
Salicylic Acid: Biodegradability and Eco-friendliness
In contrast, salicylic acid’s impact on the environment is generally considered lower. It is biodegradable, breaking down into harmless substances that do not contribute to pollution. Furthermore, advancements in its synthesis from natural sources, like willow bark, enhance its eco-friendliness.
Comparative Sustainability
When comparing the sustainability of adipic acid and salicylic acid, it’s clear that salicylic acid has a smaller environmental footprint. Its biodegradability and the potential for natural sourcing make it a more sustainable choice. In contrast, the challenge with adipic acid lies in addressing its environmental impacts, particularly in reducing greenhouse gas emissions associated with its production.

Frequently Asked Questions
What is Adipic Acid used for?
Adipic Acid serves as a key ingredient in the production of nylon and polyurethane. It is also used in the manufacture of plastics, foams, and various coatings. Beyond industrial applications, it finds a role in food as a flavorant and acidity regulator.
How does Salicylic Acid benefit the skin?
Salicylic Acid is a beta hydroxy acid that exfoliates the skin, helping to remove dead skin cells and unclog pores. It is highly effective in treating acne, reducing inflammation, and promoting a clearer, more even skin tone. Its ability to penetrate oil glands makes it a staple in skincare regimens.
Are there environmental concerns associated with Adipic Acid?
Yes, the production of Adipic Acid is associated with the emission of nitrous oxide (N2O), a potent greenhouse gas. Efforts are underway to develop more sustainable production methods to mitigate this environmental impact.
Can Salicylic Acid be used daily?
Salicylic Acid can be used daily, but its concentration and formulation should be appropriate for the skin type and condition being treated. Overuse or high concentrations can lead to skin irritation. It is advisable to start with lower concentrations and adjust usage based on skin tolerance.
Conclusion
The exploration of Adipic Acid and Salicylic Acid reveals a fascinating dichotomy between two chemical compounds, each playing integral roles in their respective domains. Their differences highlight the nuanced nature of chemical applications in our daily lives, from the clothes we wear to the products we use for skincare. The environmental and health considerations associated with each acid underscore the importance of sustainable practices and informed usage.
As we move forward, the ongoing research and development in the fields of chemistry and environmental science will likely bring forth innovations that can reduce negative impacts while enhancing the beneficial uses of these acids. Understanding their characteristics and applications not only enriches our knowledge but also empowers us to make choices that align with health and environmental sustainability.