If you are unfamiliar with the terms ketal and acetal, you may be wondering what the difference is between them. In this blog post, we will explore the key differences between ketals and acetals and how they are used in organic chemistry.
By the end of this post, you will have a better understanding of the differences between ketals and acetals.
Physical properties of ketal & acetal
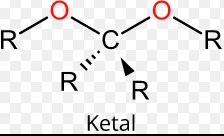
Ketal and acetal are two of the most commonly used functional groups in organic chemistry. They are both derivatives of aldehydes and both have similar molecular formulas. However, they differ in their physical properties and reactivity.
However, they differ in their physical properties and reactivity. Ketal molecules are more rigid than acetal molecules, allowing them to form stronger bonds and remain stable for longer periods of time. Additionally, ketals are more resistant to hydrolysis and are more stable than acetals.
As such, ketals are more commonly used in a variety of organic reactions. On the other hand, acetals are more reactive, making them more suitable for processes such as condensation reactions. Ultimately, the differences between ketal and acetal molecules make them useful for different types of organic reactions.
Chemical properties of ketal & acetal
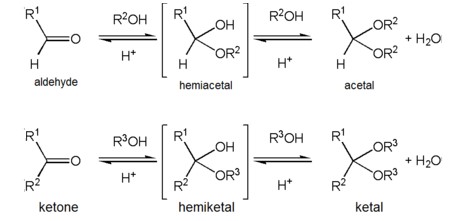
Ketals and acetals are organic compounds with very similar chemical properties. Both contain a central oxygen atom bonded to two carbon atoms and are formed from the reaction of an alcohol with an aldehyde or ketone.
This difference in structure affects the reactivity of the two compounds; ketals are more reactive than acetals, making them more prone to hydrolysis in the presence of an acidic or basic environment. This makes them useful in organic synthesis, as they can be used to protect aldehyde and ketone functional groups from unwanted reaction.
Industrial uses of ketal & acetal
Ketal and acetal are two chemical compounds that are similar in structure but have different industrial uses. Ketal is a cyclic ether with a five-member ring made of two oxygen atoms, two carbon atoms, and one oxygen-carbon bond. Acetal is a cyclic ether with a six-member ring made of three oxygen atoms, three carbon atoms, and three oxygen-carbon bonds.
Acetal is a cyclic ether with a six-member ring made of three oxygen atoms, three carbon atoms, and three oxygen-carbon bonds. Both ketal and acetal compounds can be used as catalysts in various chemical reactions, however, the main difference between the two compounds is how they are used in industrial applications. Ketal compounds are used as solvents and lubricants, while acetal compounds are used as plastics, resins, and adhesives.
Additionally, acetal compounds are more resistant to hydrolysis and have higher thermal stability, making them more suitable for use as plastics and resins.
Advantages & disadvantages of ketal & acetal
Ketal and acetal are organic compounds that have similar chemical structures but different properties that make them useful in different applications. Ketal is a cyclic ether in which two carbon atoms are linked to two oxygen atoms, while acetal is a cyclic ether with three carbon atoms linked to two oxygen atoms. Both compounds have the ability to form strong bonds with other molecules, but the differences in their structures give them different advantages and disadvantages.
Ketal is more resistant to hydrolysis, making it a better choice for applications that require prolonged stability. It is also cheaper to produce than acetal, making it a more economical choice.
On the other hand, acetal is more soluble in water, making it well-suited for applications that require enhanced solubility. It is also more reactive than ketal, making it a better choice for applications that require faster reaction times.
Ultimately, the choice between ketal and acetal will depend on the specific needs of the application.
Synthesis of ketal & acetal
Ketal and acetal are two molecules with similar chemical structures, but with some important differences. Ketal is a cyclic ether with a single oxygen atom and two methyl groups attached to it, while acetal has a single oxygen atom with two carbon atoms attached to it. The difference between the two is in the number of carbons—acetal has two carbons, while ketal has three.
This difference affects the reactivity of the molecules, as well as the type of reactions they undergo. Ketal reacts faster and with a wider range of reagents than acetal, and is used in many industrial processes.
Acetal, on the other hand, is less reactive, making it suitable for more delicate reactions. Knowing the difference between the two can help you choose the right molecule for your needs.
Final Touch
In conclusion, the difference between a ketal and an acetal is the presence of two ether oxygen atoms in a ketal and one ether oxygen atom in an acetal. Ketals are formed when aldehydes and alcohols react, while acetals are formed when ketones and alcohols react.
Ketals are stronger and more stable than acetals and can be used for a variety of applications. Acetals are also more reactive than ketals and have a wide range of uses. Both compounds can be used as solvents, plasticizers, and in the production of pharmaceuticals.