The relation between wavelength and energy is a fundamental concept that bridges the gap between physics and various scientific disciplines. This correlation is crucial in understanding how the energy associated with electromagnetic waves interacts with matter across the universe. The principle underpins technologies ranging from everyday gadgets to advanced scientific instruments, impacting numerous fields such as medicine, communication, and environmental science.
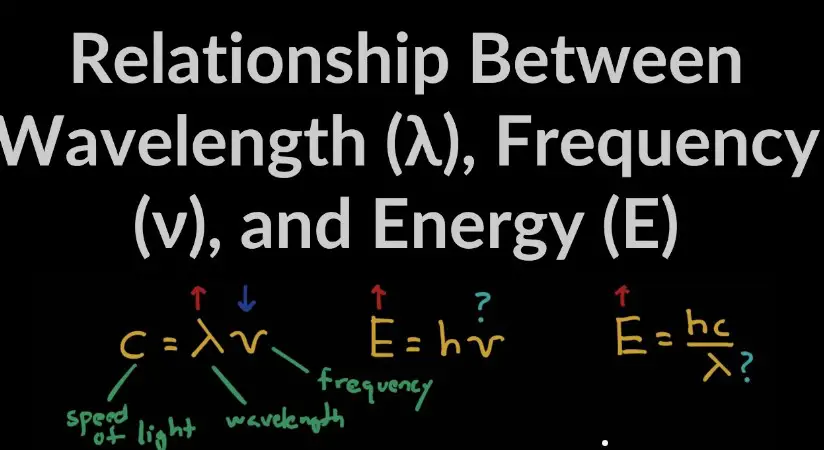
At its core, the relationship between wavelength and energy is succinctly encapsulated by Planck’s equation, which posits that the energy of a photon is directly proportional to its frequency and inversely proportional to its wavelength. This intrinsic link reveals that shorter wavelengths correspond to higher energy levels and vice versa, a principle vital for deciphering the behavior of electromagnetic radiation across its spectrum.
Delving deeper, this relationship elucidates the diverse characteristics of electromagnetic waves, from the long wavelengths of radio waves to the short wavelengths of gamma rays. Each portion of the electromagnetic spectrum plays a unique role in our understanding of the universe and in the development of technology, highlighting the significance of the wavelength-energy relationship in both theoretical physics and practical applications.
Basics of Wavelength
Definition and Explanation
Wavelength refers to the distance between consecutive peaks or troughs in a wave. In the context of electromagnetic waves, this distance determines much of the wave’s properties, including its energy. Wavelength is a fundamental concept in physics, underpinning the behavior of light and all forms of electromagnetic radiation.
Units of Measurement
The primary unit for measuring wavelength is the meter (m), although wavelengths can range vastly in size. As a result, subunits like nanometers (nm) for very short wavelengths, such as those of visible light, and kilometers (km) for longer wavelengths, such as radio waves, are commonly used.
Role in Electromagnetic Spectrum
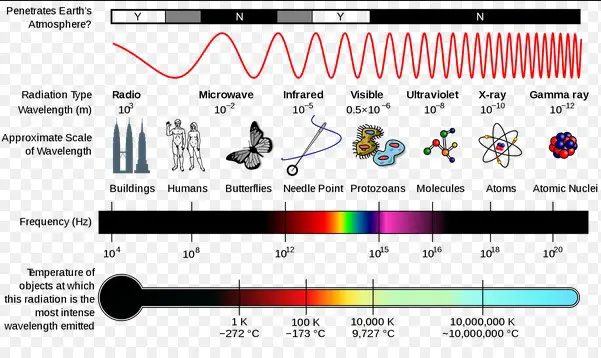
Wavelength categorizes the electromagnetic spectrum into various types of radiation, including radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays. Each type of radiation has a specific wavelength range, influencing how it interacts with matter and its applications in technology and science.
Basics of Energy
Definition and Explanation
Energy in the context of electromagnetic waves is the capacity to do work or cause change. It’s intrinsically linked to the wave’s frequency and wavelength, determining how the wave can interact with physical objects, from heating substances to transmitting information.
Units of Measurement
The energy of electromagnetic waves is commonly measured in joules (J). However, when discussing photons—the particles of light—energy is often measured in electronvolts (eV), a unit more suited to the quantum scale of interactions.
Different Forms of Energy
Electromagnetic waves carry radiant energy, which can transform into other energy types upon interaction with matter, such as thermal energy (heat), chemical energy, and electrical energy. This transformation underlies the vast applications of electromagnetic waves in various fields.
Electromagnetic Spectrum Overview
Description of the Electromagnetic Spectrum
The electromagnetic spectrum encompasses all types of electromagnetic radiation, differentiated by their wavelengths. It ranges from very long radio waves to extremely short gamma rays. Despite these differences, all electromagnetic waves travel at the speed of light in a vacuum.
Types of Electromagnetic Waves
- Radio Waves: Used in communication technologies.
- Microwaves: Employed in radar and cooking appliances.
- Infrared: Key in heating and remote control technologies.
- Visible Light: The only part of the spectrum visible to the human eye.
- Ultraviolet: Has applications in sterilization and fluorescence.
- X-rays: Crucial in medical imaging.
- Gamma Rays: Used in cancer treatment and radioactive decay processes.
Wavelength Ranges and Their Applications
Each type of electromagnetic wave, due to its unique wavelength range, has specific applications exploiting its energy properties, from the long wavelengths of radio waves used in broadcasting to the short wavelengths of gamma rays utilized in medical therapies.
Fundamental Principles
Wave-Particle Duality
Electromagnetic radiation exhibits dual behavior, acting both as waves and as particles (photons). This principle is central to quantum mechanics, explaining phenomena like photon absorption and emission by atoms.
Speed of Light and Its Significance
The speed of light in a vacuum, approximately 299,792 kilometers per second (km/s), is a fundamental constant of nature. It signifies the maximum speed at which all electromagnetic waves propagate, affecting their energy and wavelength.
Wavelength vs. Energy
Direct Relationship Explanation
Wavelength and energy are inversely related in electromagnetic waves; as the wavelength decreases, the energy increases. This relationship is crucial for understanding how different types of electromagnetic radiation interact with the environment and matter.
Mathematical Formula Derivation
Planck’s equation provides the mathematical foundation for the relationship between wavelength and energy: �=ℎ��E=λhc, where �E is energy, ℎh is Planck’s constant, �c is the speed of light, and �λ is the wavelength. This equation highlights the inverse relationship between energy and wavelength.
Practical Examples
- X-rays have short wavelengths and high energy, enabling them to penetrate human tissue for imaging.
- Radio waves, with their long wavelengths and lower energy, are ideal for broadcasting over long distances without causing harm to biological tissues.
Factors Influencing Relationship
Medium’s Role
Different mediums can affect the speed at which light travels, indirectly influencing the relationship between wavelength and energy. For instance, light moves slower in water than in a vacuum, altering its wavelength but not its frequency, showing the medium’s impact on this fundamental relationship.
Temperature Effects
Temperature can significantly affect the energy levels within a system, impacting the emission and absorption spectra of materials. Higher temperatures generally increase energy, affecting the wavelengths emitted or absorbed by substances, demonstrating a direct temperature-wavelength correlation.
Quantum Mechanics Insights
Quantum mechanics offers a deeper understanding of how particles and waves behave at microscopic levels. Insights from quantum mechanics, like the Heisenberg uncertainty principle, reveal the intricate interplay between wavelength, momentum, and energy, showcasing the non-intuitive aspects of their relationship.
Applications in Real World
Communications Technology
The choice of wavelength in communications technology directly impacts signal range and penetration. Longer wavelengths, for example, are used in radio and television broadcasts due to their ability to travel longer distances and penetrate obstacles more effectively.
Medical Imaging and Treatments
In medical imaging, different energy wavelengths are utilized for diverse purposes. X-rays, with their short wavelengths, offer high-energy imaging capabilities essential for seeing inside the human body, while lower-energy waves may be used in MRI scans to avoid ionizing radiation.
Renewable Energy Sources
Solar panels are designed to capture specific wavelengths of sunlight, converting them into electricity. Understanding the spectrum of sunlight and its energy content is crucial for optimizing solar cell efficiency and developing new renewable energy technologies.
Measurement Techniques
Spectroscopy
Spectroscopy is a critical technique for measuring the wavelengths and energy of electromagnetic radiation, widely used in chemistry, physics, and astronomy to analyze the composition of materials and celestial objects.
Photometry
Photometry involves measuring the intensity of light as perceived by the human eye. It’s used in designing lighting solutions and in astronomical observations to estimate the distances and properties of stars based on their apparent brightness.
Practical Challenges and Solutions
Measuring wavelengths and energy presents several challenges, such as calibrating instruments and accounting for environmental variables. Advances in technology and methodology have led to innovative solutions, improving the accuracy and reliability of these measurements.
Advances and Innovations
Recent Scientific Research
Recent research in photonics and quantum physics continues to unveil new aspects of the wavelength-energy relationship, leading to potential new materials and technologies that could revolutionize industries from computing to telecommunications.
Technological Breakthroughs
Technological breakthroughs, such as the development of lasers and quantum dots, have been founded on the precise manipulation of wavelength and energy. These innovations offer unprecedented control over light, opening up new applications in medicine, computing, and material science.
Future Prospects
The future holds promising prospects for further exploration of the wavelength-energy relationship, with potential advancements in energy storage, quantum computing, and telecommunications. As our understanding deepens, so too will our ability to harness this relationship for innovative solutions to complex problems.
Frequently Asked Questions
What is Planck’s equation?
Planck’s equation, a cornerstone of quantum mechanics, describes the quantized nature of energy. It states that the energy (E) of a photon is equal to its frequency (f) multiplied by Planck’s constant (h), establishing a direct proportionality between energy and frequency, and thus an inverse relationship with wavelength. This equation is pivotal in explaining phenomena such as the photoelectric effect and blackbody radiation.
How does wavelength affect energy?
Wavelength and energy are inversely related; as the wavelength of an electromagnetic wave decreases, its energy increases. This relationship is crucial in applications ranging from medical imaging, where shorter wavelengths offer greater energy and penetration capabilities, to telecommunications, where longer wavelengths are utilized for their ability to travel greater distances.
Why is the wavelength-energy relationship important?
The wavelength-energy relationship is foundational in understanding electromagnetic radiation’s interaction with matter. It informs the design and application of technologies across a spectrum of fields, including spectroscopy, communication, and astrophysics. By comprehending how wavelength influences energy, scientists can tailor electromagnetic waves for specific purposes, from treating cancer with targeted radiation therapy to exploring the cosmos through various wavelengths.
Conclusion
The intricate dance between wavelength and energy underpins much of modern science and technology. This relationship not only deepens our understanding of the physical world but also drives innovation in countless applications that affect our daily lives. From the vastness of space explored through different wavelengths of light to the precision of medical diagnostics achieved with specific energy levels, the impact of this fundamental principle is both profound and far-reaching.
As we continue to explore and harness the power of electromagnetic radiation, the knowledge of how wavelength and energy interplay will remain a cornerstone of scientific advancement. The applications and implications of this relationship are as limitless as the spectrum itself, promising new discoveries and technologies that will shape our future.