Temperature and Pressure are two of the most important physical variables used in many industries and scientific fields. In this blog, we will discuss the relation between temperature and pressure, including the impact on various materials, and the applications of this knowledge in engineering and science. We will also discuss how changes in temperature and pressure can be used to manipulate the properties of materials, and the implications this has for different industries.
We will also discuss how changes in temperature and pressure can be used to manipulate the properties of materials, and the implications this has for different industries.
Historical perspective
Temperature and pressure have a fascinating relationship that has been studied and discussed for centuries. In 1662, Robert Boyle famously discovered that when the temperature of a gas was increased, the pressure of the gas also increased. This was the first time a connection between temperature and pressure had been formally documented, and it sparked a scientific revolution in our understanding of thermodynamics.
Today, we understand that the two are inextricably linked and that an increase in temperature results in an increase in pressure, and vice versa. This relationship is incredibly important in many applications, from engineering to meteorology, and is a crucial factor in our understanding of the universe.
Temperature and pressure relationship
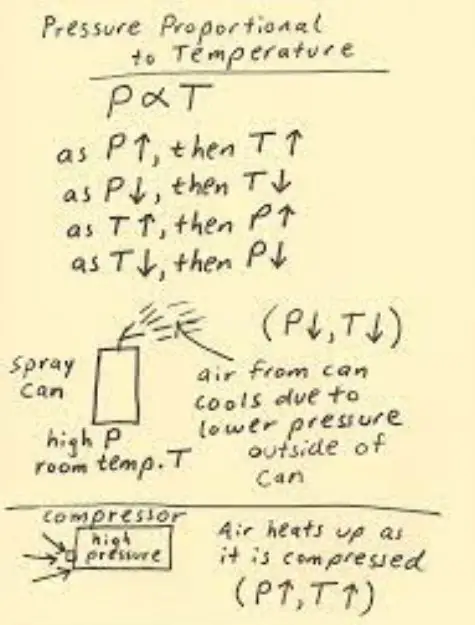
The relationship between temperature and pressure is an interesting one. As temperature increases, so does pressure – and vice versa.
In other words, as the temperature increases, so does the pressure – and as the temperature decreases, so does the pressure. This law explains why the pressure in a tire changes as the temperature changes – the higher the temperature, the higher the pressure.
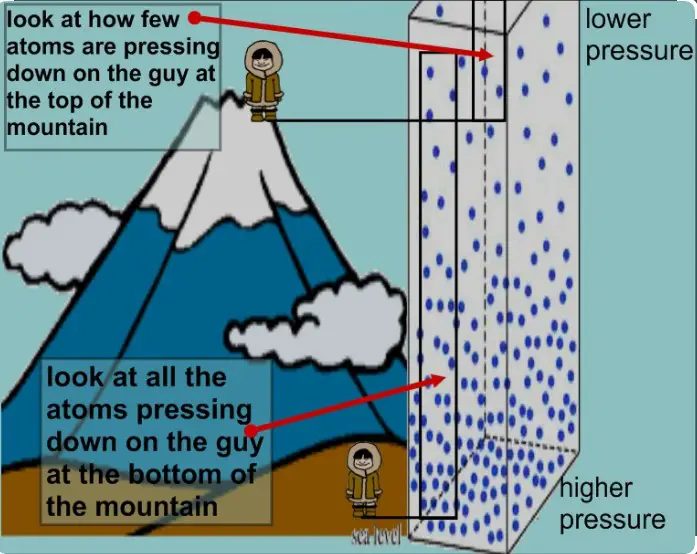
It also helps explain why the atmospheric pressure decreases with altitude – the higher you go, the lower the temperature. Knowing this relationship is essential for scientists and engineers who need to know how temperature and pressure interact with each other.
Factors affecting the relationship
The relationship between temperature and pressure is an important factor in many scientific and industrial applications. Temperature is directly related to the pressure exerted by a given volume of gas. As temperature increases, the pressure exerted by the gas also increases, and vice versa.
As temperature increases, the pressure exerted by the gas also increases, and vice versa. This phenomenon can be explained by the fact that when the temperature of a gas increases, the molecules of the gas move faster and collide more frequently, resulting in a higher pressure. Conversely, when the temperature of a gas decreases, the molecules move slower and collisions between them are less frequent, resulting in a lower pressure.
Understanding this relationship can be very important for scientists and engineers, as it helps them make more accurate predictions and decisions.
Applications of the relationship
The relationship between temperature and pressure has many practical applications, from the functioning of everyday household appliances to the complex mechanics of aircraft engines. In the home, the most common application of the temperature-pressure relationship is the operation of a refrigerator. The refrigerant is compressed and cooled, and the expansion of the gas as it warms causes a drop in pressure that helps draw heat out of the refrigerator’s interior.
The refrigerant is compressed and cooled, and the expansion of the gas as it warms causes a drop in pressure that helps draw heat out of the refrigerator’s interior. The same principle applies to air conditioners, heat pumps, and other temperature control systems. In automotive engines, the temperature-pressure relationship helps to regulate fuel combustion and increase engine performance.
In aircraft engines, the temperature-pressure relationship is used to control the speed at which the engine runs and helps to maximize fuel efficiency. In all of these applications, the relationship between temperature and pressure is essential for efficient operation.
Theoretical models
The relationship between temperature and pressure is an important consideration for many scientific models. Temperature and pressure are two of the most fundamental properties of any system, and their relationship is a major factor in determining the behavior of that system.
The theoretical models used to comprehend this relationship are based on the laws of thermodynamics, which describe how heat, pressure, and temperature interact with each other. By understanding the relationship between temperature and pressure, scientists can better understand the behavior of a system, and even predict how changes in one will affect the other. This knowledge is invaluable for creating effective models that can be used to accurately predict future results.
Conclusion
In conclusion, the relationship between temperature and pressure is an important one and can be observed in many different areas of science and engineering. As temperature increases, so does the pressure, and vice versa.
This relationship is essential to understand in order to properly design and operate any device that needs to be temperature- and/or pressure-controlled. Furthermore, a change in temperature can also have an effect on the equilibrium state of a system, thus affecting the pressure as well. In any case, understanding the relationship between temperature and pressure is a necessity for anyone involved with the design and operation of temperature and pressure-controlled systems.
