Acid-base chemistry sits at the heart of our understanding of chemical reactions, influencing everything from biological systems to industrial processes. The concepts of acid dissociation constant (Ka) and base dissociation constant (Kb) are pivotal in quantifying the strength of acids and bases, respectively. These constants provide a framework for predicting the behavior of substances in various environments, making them essential tools for chemists and researchers.
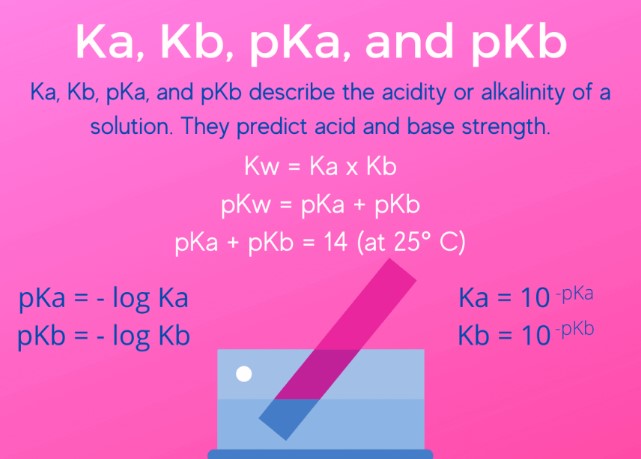
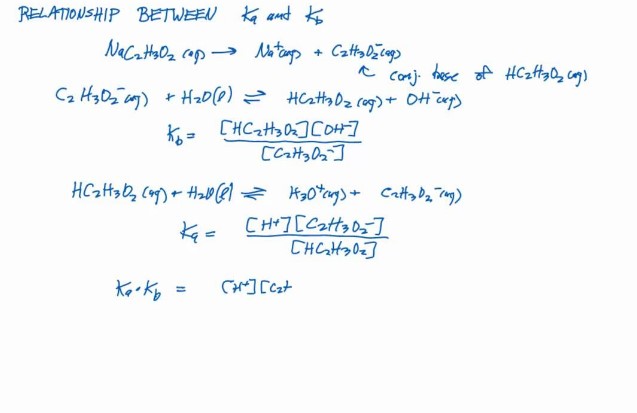
The relation between Ka and Kb is not just a matter of academic interest but a foundational aspect of chemical equilibrium in aqueous solutions. For any acid-base conjugate pair in water, the product of their dissociation constants (Ka for the acid and Kb for the base) is constant and equal to the water ionization constant (Kw). This relationship allows for the determination of one constant if the other is known, facilitating a deeper understanding of acid-base interactions.
Focusing on the Ka and Kb of substances reveals their intrinsic properties and predicts their behavior in solution. These constants guide the formulation of drugs, the manufacture of materials, and the protection of the environment. By exploring their interrelation, we not only grasp the fundamentals of acid-base chemistry but also unlock the potential to innovate and solve complex problems in science and technology.
Acid-Base Theories
Understanding the behavior of acids and bases is foundational to chemistry. Over the years, scientists have developed several theories to explain how these substances interact, especially in water.
Acid-Base Definitions
Arrhenius Theory
Svante Arrhenius introduced a straightforward idea in the late 19th century. According to the Arrhenius theory, acids are substances that increase the concentration of hydrogen ions (H+) in water, while bases increase the concentration of hydroxide ions (OH-). This theory was groundbreaking because it linked acid-base behavior to ions, fundamental particles in chemistry.
Brønsted-Lowry Theory
Years later, Johann Brønsted and Thomas Lowry proposed a broader definition. They focused not just on water but on the transfer of protons. A Brønsted-Lowry acid is a proton donor, and a Brønsted-Lowry base is a proton acceptor. This theory expanded the list of substances that could be considered acids or bases beyond those soluble in water.
Lewis Theory
Gilbert N. Lewis went a step further, offering a more generalized approach. In the Lewis theory, an acid is an electron pair acceptor, and a base is an electron pair donor. This concept opened the door to understanding acid-base reactions in non-aqueous solutions and even reactions where no protons are transferred.
Acids and Bases in Water
Water, the solvent of life, plays a crucial role in acid-base chemistry due to its unique properties.
Role of Water as a Solvent
Water is a universal solvent because of its ability to dissolve a vast array of substances. Its polar nature allows it to engage in hydrogen bonding, making it an excellent medium for acid-base reactions. Water can act as both an acid (proton donor) and a base (proton acceptor), showcasing its amphiprotic nature.
Ionization Process
When acids or bases dissolve in water, they undergo ionization. This process is essential for conducting electricity and participating in chemical reactions. The degree of ionization impacts the substance’s strength as an acid or base.
Ka: Acid Dissociation Constant
Definition of Ka
The acid dissociation constant (Ka) measures an acid’s strength by quantifying its tendency to donate a proton to the solvent. Ka is a crucial concept in understanding the reactivity and behavior of acids in solution.
Explanation of Acid Dissociation
Acid dissociation is the process where an acid releases a proton, resulting in the formation of its conjugate base. This equilibrium between the acid, its conjugate base, and the hydrogen ion concentration is represented by Ka.
Mathematical Expression of Ka
The mathematical expression for Ka is defined as the product of the concentrations of the hydrogen ions and the conjugate base, divided by the concentration of the acid. Mathematically, it’s shown as:
��=[�+][�−][��]Ka=[HA][H+][A−]
where [�+][H+] is the concentration of hydrogen ions, [�−][A−] is the concentration of the conjugate base, and [��][HA] is the concentration of the acid.
Factors Affecting Ka
Several factors can influence the value of Ka, indicating how different conditions affect acid strength.
Molecular Structure
The molecular structure of an acid plays a significant role in its ability to donate protons. Elements such as electronegativity and the stability of the conjugate base can significantly impact Ka.
Solvent Effect
The solvent in which the acid is dissolved can also affect its dissociation. Solvents that can stabilize ions well tend to increase Ka values.
Temperature
Temperature changes can influence the ionization energy and thus affect the Ka. Typically, an increase in temperature leads to an increase in the value of Ka.
Kb: Base Dissociation Constant
Definition of Kb
The base dissociation constant (Kb) mirrors Ka but for bases. It measures a base’s strength by its ability to accept a proton, quantifying the equilibrium between the base, its conjugate acid, and the hydroxide ion concentration.
Explanation of Base Dissociation
Base dissociation refers to the process where a base accepts a proton from water, producing its conjugate acid and hydroxide ions. The strength of a base can be gauged by its Kb value.
Mathematical Expression of Kb
Kb is calculated similarly to Ka but focuses on the base’s reaction with water. It’s given by:
��=[��+][��−][�]Kb=[B][BH+][OH−]
where [��+][BH+] is the concentration of the conjugate acid, [��−][OH−] is the concentration of hydro

pH and pOH
The concepts of pH and pOH are central to understanding acid-base chemistry. They provide a measure of the acidity or basicity of a solution, which is crucial for a wide range of scientific and practical applications.
Definitions and Importance
Explanation of pH and pOH
- pH is a scale used to specify the acidity or basicity of an aqueous solution. It is the negative logarithm of the concentration of hydrogen ions (�+H+) in a solution.
- pOH, on the other hand, measures the basicity of a solution through the negative logarithm of the concentration of hydroxide ions (��−OH−).
- Both scales range from 0 to 14, with 7 being neutral. A pH less than 7 indicates acidity, while a pH greater than 7 indicates alkalinity. The same is true for pOH but in the opposite direction.
Their Role in Acid-Base Chemistry
pH and pOH are instrumental in predicting the direction of acid-base reactions, understanding buffer solutions, and calculating the equilibrium constants for these reactions. They serve as a quick reference for assessing the corrosive or scaling potential of water, crucial in many industrial and environmental contexts.
Connecting pH, pOH, Ka, and Kb
Understanding the interplay between pH, pOH, Ka, and Kb is essential for comprehending the equilibrium states of acid-base reactions in aqueous solutions.
Calculations Involving pH, pOH, Ka, and Kb
- To find pH from Ka, you first calculate the concentration of �+H+ ions using the formula for Ka and then apply the pH formula.
- Similarly, pOH can be found from Kb by calculating the concentration of ��−OH− ions and applying the pOH formula.
- The relationship between pH and pOH can be summarized as ��+���=14pH+pOH=14, which is based on the water ionization constant (��Kw) at 25°C.
Practical Examples
- Acetic Acid in Vinegar: Calculating the pH of vinegar involves using the Ka value for acetic acid to find the concentration of �+H+ ions.
- Ammonia Solutions: Determining the pOH of an ammonia solution requires the Kb value for ammonia to calculate the concentration of ��−OH− ions.
Applications in Real World
The principles of acid-base chemistry, especially those involving pH, pOH, Ka, and Kb, find applications in a variety of real-world scenarios.
Industrial Applications
Use in Manufacturing Processes
In the chemical industry, controlling the pH is crucial for the desired reaction outcomes. For example, in the synthesis of pharmaceuticals, a specific pH range ensures the effectiveness and safety of drugs.
Quality Control in the Food Industry
The acidity or basicity of food products affects their taste, preservation, and safety. Monitoring and adjusting the pH is key to maintaining quality and extending shelf life.
Environmental Science
Acid Rain
Understanding the pH of rainwater helps identify acid rain, which results from the dissolution of atmospheric pollutants. This knowledge is essential for developing strategies to mitigate its environmental impact.
Ocean Acidification
The increasing acidity of oceans, measured through pH, is a significant concern. It affects marine life and ecosystems, highlighting the importance of understanding pH in environmental conservation.
Health and Medicine
Drug Formulation
The pH of the environment influences the stability and absorption of medications. Formulating drugs requires careful consideration of these factors to ensure their efficacy and safety.
Gastric Acid Analysis
Analyzing the pH of gastric acid can help diagnose and treat digestive disorders. It provides insights into the stomach’s function and its impact on overall health.
Common Misunderstandings
Clarifying misconceptions about Ka, Kb, and pH is crucial for a deeper understanding of acid-base chemistry.
Ka, Kb, and Strength
- The strength of an acid or base is not solely determined by high Ka or Kb values. It’s also influenced by the solvent and the specific reaction conditions.
- A common misunderstanding is that a substance with a higher Ka or Kb is always stronger under all conditions, which is not the case.
Misinterpretation of pH
- pH is often mistakenly considered a direct indicator of acid strength. However, pH also depends on the concentration of the acid or base in solution.
- A common pitfall is assuming that a solution with a higher pH is always less acidic without considering the dilution factor.
Frequently Asked Questions
What is Ka in chemistry?
Ka, or the acid dissociation constant, measures the strength of an acid in solution. It quantifies the extent to which an acid donates its proton to the solvent, typically water. A higher Ka value indicates a stronger acid, meaning it readily releases its proton, leading to a greater concentration of hydronium ions in the solution.
How does Kb relate to base strength?
Kb, the base dissociation constant, reflects a base’s strength by indicating how effectively it can accept a proton from the solvent. A base with a higher Kb value is considered stronger, as it is more likely to attract a proton, increasing the concentration of hydroxide ions in the solution. This makes Kb an essential parameter in assessing a base’s reactivity and its ability to neutralize acids.
Why is the product of Ka and Kb constant?
The product of Ka and Kb is constant for a given acid-base conjugate pair in water because it equals the water ionization constant (Kw). This relationship is grounded in the principle of chemical equilibrium, where the forward and reverse reactions occur at the same rate. In aqueous solutions, the dissociation of water into hydronium and hydroxide ions sets a dynamic balance that links the strengths of conjugate acids and bases.
How do pH and pOH relate to Ka and Kb?
pH and pOH are measures of the acidity and basicity of a solution, respectively. They are logarithmically related to the concentrations of hydronium and hydroxide ions. Since Ka and Kb determine these ion concentrations for acids and bases, pH and pOH can be calculated using the dissociation constants. This connection allows for the assessment of a solution’s acid-base character through its pH and pOH values.
Conclusion
Exploring the relationship between Ka and Kb unveils the intricate balance of acids and bases in solution, a cornerstone of chemical science that influences a myriad of processes in both nature and industry. This understanding not only deepens our grasp of fundamental chemistry but also enhances our ability to manipulate chemical reactions for desired outcomes. It serves as a reminder of the elegance and complexity of the molecular world, where even seemingly simple concepts like acid and base strength hold the keys to unlocking vast scientific and practical applications.
As we continue to investigate and apply the principles of acid-base chemistry, the insights gained from the relationship between Ka and Kb will undoubtedly contribute to advancements in various fields. From developing new medications to safeguarding our environment, the practical implications of this knowledge are as profound as they are far-reaching, underscoring the importance of chemistry in solving the challenges of today and tomorrow.