Thermodynamics sits at the heart of understanding how energy is transformed and conserved within a system, especially when it comes to the study of gases. Central to this field are two crucial concepts: Cp (heat capacity at constant pressure) and Cv (heat capacity at constant volume). These parameters not only reveal how a substance responds to changes in temperature but also encapsulate fundamental principles of energy flow and storage.
The relationship between Cp and Cv is a cornerstone of thermodynamic theory, offering insights into the behavior of gases under different conditions. In essence, Cp represents the amount of heat energy required to raise the temperature of a substance by one degree Celsius at constant pressure, whereas Cv is the amount of heat energy required under constant volume. Their interplay is vital for predicting and understanding the energy dynamics in various physical and engineering processes.
This topic encapsulates a wide array of applications, from the design of engines and refrigeration systems to the prediction of climate patterns. Understanding the differences and connection between Cp and Cv not only deepens our comprehension of thermodynamic systems but also enhances our ability to optimize processes for energy efficiency and sustainability. The depth of knowledge in this area continues to influence technological advancements and environmental strategies, marking its importance in both academic research and practical engineering.

Basics of Thermodynamics
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, radiation, and physical properties of matter. The fundamental principles of thermodynamics govern the operation of engines, refrigerators, and many other devices we use in our daily lives.
Heat Capacity
Heat capacity is a measure of the amount of heat energy required to raise the temperature of a given substance by a certain temperature interval. It is an intrinsic property of the material, indicating how much heat is absorbed or released as the substance’s temperature changes. Heat capacity can be expressed as either a specific heat capacity, which is heat capacity per unit mass of a substance, or as molar heat capacity, which is heat capacity per mole of a substance.
Internal Energy
The internal energy of a system refers to the total energy contained within it, attributed to the kinetic and potential energies of the molecules. The kinetic energy arises from the motion of the molecules, while the potential energy is related to the molecular structure and the interactions between molecules. Changes in internal energy can result from heat transfer, work done by or on the system, and mass flow into or out of the system.
First Law of Thermodynamics
The First Law of Thermodynamics, also known as the Law of Energy Conservation, states that energy cannot be created or destroyed in an isolated system. The change in internal energy of a system is equal to the heat added to the system minus the work done by the system on its surroundings. Mathematically, it is expressed as:
Δ�=�−�ΔU=Q−W
where Δ�ΔU is the change in internal energy, �Q is the heat added to the system, and �W is the work done by the system.
Cp and Cv Explained
Understanding Cp and Cv requires diving into how gases react under different conditions of pressure and volume.
Definition of Cp
Cp stands for the heat capacity at constant pressure. It is the amount of heat required to increase the temperature of a unit mass of a substance by one degree Celsius while keeping the pressure unchanged. Cp is particularly relevant in processes where the pressure remains constant, such as in atmospheric conditions.
Definition of Cv
Cv stands for the heat capacity at constant volume. It represents the amount of heat required to raise the temperature of a unit mass of a substance by one degree Celsius without any change in volume. Cv is crucial for understanding the behavior of gases in enclosed spaces where the volume is fixed.
Physical Interpretation
The physical interpretation of Cp and Cv lies in their ability to provide insights into the energy storage and heat transfer capabilities of substances under various conditions. They reflect how substances absorb heat and convert it into internal energy, leading to temperature changes. These properties are fundamental in designing and analyzing systems where heat transfer and temperature changes are involved, such as engines and refrigerators.
Key Differences
Cp and Cv highlight different aspects of a substance’s behavior under varying conditions.
Volume vs. Pressure
The key difference between Cp and Cv is the constraint under which the heat capacity is measured: Cp under constant pressure and Cv under constant volume. This distinction is critical because the work done by or on the system affects the internal energy differently in each case, leading to different heat requirements for the same temperature change.
Energy Transfer Methods
Another difference lies in the energy transfer methods associated with Cp and Cv. Under constant pressure, the system can do work by expanding, which is not the case under constant volume. Therefore, Cp is generally higher than Cv, as part of the energy supplied at constant pressure is used for work against the external pressure, in addition to increasing the internal energy.
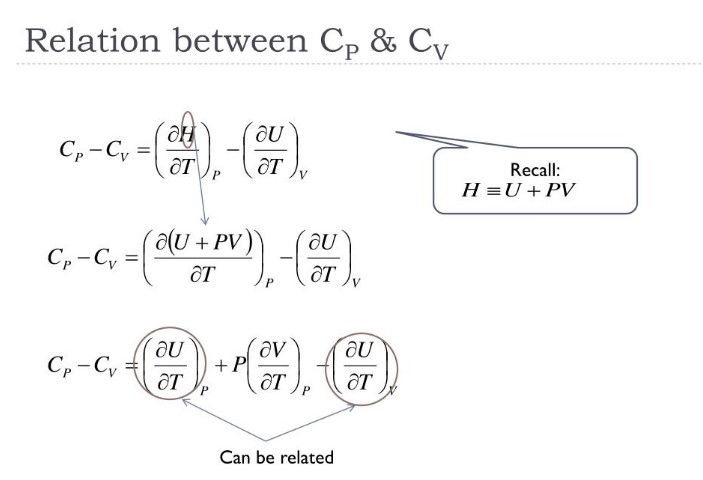
Mathematical Relationship
The mathematical relationship between Cp and Cv is a fundamental aspect of thermodynamics that connects these properties with the gas constant and the nature of the substance, especially for ideal gases.
Formula Derivation
For an ideal gas, the relationship between Cp and Cv is given by the equation:
��−��=�Cp−Cv=R
where �R is the universal gas constant. This equation is derived from the first law of thermodynamics, considering the work done during expansion or compression at constant pressure or volume.
Role of Gas Constant
The gas constant �R is a bridge between the macroscopic and microscopic properties of gases, relating volume, pressure, temperature, and the number of moles in the ideal gas equation. It plays a crucial role in linking Cp and Cv to the behavior of ideal gases.
Specific Heats for Ideal Gases
For ideal gases, Cp and Cv are not just properties of the substance but also reflect the gas’s molecular structure and degrees of freedom. Monatomic gases, diatomic gases, and polyatomic gases have different specific heats because of their varying molecular complexities and ways of storing internal energy.
Factors Influencing Cp and Cv
Understanding how different factors affect Cp and Cv is crucial for accurate predictions and analyses in various scientific and engineering domains.
Temperature Dependence
Both Cp and Cv are not static values; they vary with temperature. For many substances, especially gases, the heat capacity increases with temperature. This is due to the increased kinetic energy of molecules, which leads to greater internal energy changes for a given heat input. However, this relationship can be complex, influenced by molecular structure and the specific heat capacity of the substance.
Molecular Structure
The molecular structure of a substance greatly impacts its heat capacity. Simple monatomic gases like helium have lower Cp and Cv values compared to more complex polyatomic gases like methane. This is because polyatomic gases have more degrees of freedom, such as vibration and rotation, allowing them to store more internal energy.
Phase Changes
Phase changes also play a significant role in altering Cp and Cv. During a phase transition, such as melting or boiling, a substance absorbs or releases a significant amount of heat without a temperature change. This latent heat is a critical factor in determining the overall heat capacity of substances during phase transitions.
Practical Applications
The concepts of Cp and Cv find applications in various fields, from engineering to environmental science, illustrating their importance beyond theoretical physics.
Engineering and Design
In engineering and design, knowledge of Cp and Cv is essential for the development of efficient thermal systems, such as engines, heat exchangers, and HVAC systems. Designers use these values to calculate the energy required for heating and cooling processes, ensuring that systems are both effective and efficient.
Energy Efficiency
Improving energy efficiency in industrial processes often involves optimizing the use of heat and work. Understanding the specific heat capacities of substances involved allows engineers to design processes that minimize energy losses, leading to more sustainable and cost-effective operations.
Climate Modeling
In climate modeling, Cp and Cv are key parameters for simulating the Earth’s atmosphere and oceans’ thermal dynamics. These models help predict how heat is distributed around the planet, which is crucial for understanding and forecasting climate change.
Calculating Cp and Cv
Determining the heat capacity of substances can be done through various experimental and theoretical methods, each with its own set of challenges and advantages.
Experimental Methods
Experimental determination of Cp and Cv involves measuring the temperature change of a substance when a known amount of heat is added or removed. These methods require precise instruments to measure temperature changes and controlled environments to maintain constant pressure or volume.
Theoretical Approaches
Theoretical approaches to calculating Cp and Cv often involve statistical mechanics and quantum chemistry. These methods use the molecular structure and interactions within a substance to predict heat capacity. While complex, they provide valuable insights, especially for substances where experimental measurements are difficult.
Examples and Case Studies
Real-world applications and case studies demonstrate the practical significance of accurately determining Cp and Cv. For instance, in designing a new refrigerant for air conditioning systems, both experimental and theoretical analyses of Cp and Cv help in selecting compounds with optimal thermal properties.
Advanced Topics
Delving deeper into thermodynamics, the behavior of non-ideal gases and the specifics of polyatomic gases reveal the nuances of real-world systems.
Non-Ideal Gases
For non-ideal gases, deviations from the ideal gas law become significant, especially under high pressure or low temperature. These deviations affect the relationship between Cp and Cv, necessitating corrections based on real gas behavior, described by equations of state like the Van der Waals equation.
Polyatomic Gases
Polyatomic gases exhibit complex behavior due to their internal degrees of freedom, such as vibrations and rotations. Understanding how these modes contribute to heat capacity is crucial for accurate predictions in chemical engineering and atmospheric science.
Real-World Deviations
Real-world deviations from theoretical models underscore the importance of empirical measurements and tailored theories. Factors like impurities, external fields, and phase transitions can significantly affect Cp and Cv, highlighting the need for precise measurements and adaptive models in practical applications.
Frequently Asked Questions
What is Heat Capacity?
Heat capacity is a physical property of a substance that measures the amount of heat required to change its temperature by a given amount. It reflects the substance’s ability to store and release heat, playing a crucial role in thermodynamics and heat transfer processes.
Why are Cp and Cv important in Thermodynamics?
Cp and Cv are essential in thermodynamics because they help describe how a gas responds to temperature changes under constant pressure and volume, respectively. This information is critical for predicting the behavior of gases in various conditions and for designing systems that involve heat transfer and energy conversion.
How do Cp and Cv differ for different substances?
Cp and Cv values can significantly vary among different substances due to their molecular structures and interactions. For instance, gases generally have higher Cp and Cv values than solids and liquids, and the gap between Cp and Cv widens for gases with more complex molecular structures.
Can the relationship between Cp and Cv change?
The relationship between Cp and Cv is influenced by the specific gas law that applies to a substance and can change with temperature, pressure, and the gas’s phase. However, for ideal gases, the relationship is constant and described by the specific heat ratio, a fundamental constant in thermodynamics.
Conclusion
Exploring the relationship between Cp and Cv unveils a deeper understanding of how substances interact with energy, highlighting the intricate balance between temperature, pressure, and volume in thermodynamic systems. These concepts not only form the foundation of thermal physics but also guide the design and innovation of countless engineering solutions, from improving energy efficiency to advancing sustainable technology practices.
As we continue to push the boundaries of science and engineering, the principles underlying Cp and Cv will remain pivotal in shaping our approach to energy management and environmental stewardship. By harnessing this knowledge, we can craft more efficient systems and contribute to a more sustainable future, demonstrating the enduring relevance of thermodynamics in our quest to understand and optimize the world around us.
