Surface chemistry, an essential branch of chemical science, explores how chemical processes at interfaces differ from those in bulk materials. One crucial aspect of this field is the study of zeta potential and point of zero charge (PZC), which are pivotal in understanding how substances interact at molecular levels. These concepts play significant roles in a myriad of industrial and environmental processes, affecting everything from water purification to medicine development.
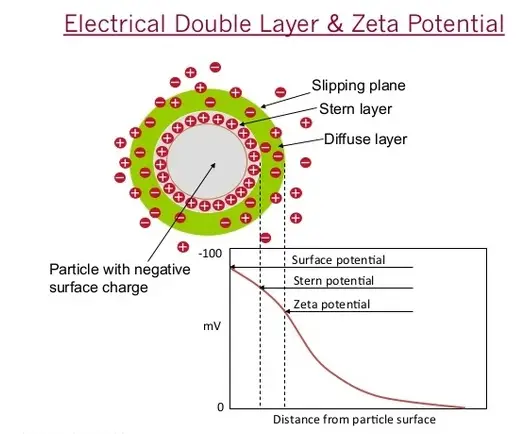
Zeta potential is an electrokinetic measure that indicates the stability of colloidal dispersions, reflecting the effective charge a particle acquires in a specific medium. In contrast, the point of zero charge refers to the pH at which a surface carries no net electrical charge. Though both relate to surface charges, they differ significantly in their implications and measurement techniques, making their distinction crucial for practical applications.
The differences between zeta potential and PZC are not just academic; they have practical implications in various fields, including water treatment, pharmaceuticals, and advanced material manufacturing. A clear understanding of these differences helps scientists and engineers tailor approaches to optimize product performance and environmental compliance.
Zeta Potential Basics
Definition and Significance
Zeta potential is a scientific measure representing the electrical difference between the dispersion medium and the stationary layer of fluid surrounding a particle. This metric is critical in colloid chemistry, as it indicates the stability of colloidal dispersions. The higher the absolute value of the zeta potential, the more likely the particles are to repel each other, which prevents them from aggregating. This stability is essential in industries ranging from pharmaceuticals, where it affects the efficacy of drug delivery systems, to environmental engineering, where it influences the treatment of wastewater.
Measurement Techniques
The measurement of zeta potential is typically performed using techniques such as:
- Electrophoresis: Particles are subjected to an electric field, and their movement speed indicates their zeta potential.
- Electroacoustic Spectroscopy: This method measures the sound waves produced by oscillating particles in an electric field to determine their zeta potential.
- Streaming Potential: This approach evaluates the voltage generated when a liquid is forced through a packed bed of particles.
Each technique offers different advantages depending on the sample’s nature and the precision required.
Factors Influencing Zeta Potential
Several factors can affect the zeta potential of particles:
- pH of the medium: Changes in pH can alter the surface charge of particles, thereby affecting their zeta potential.
- Ionic strength: Higher concentrations of ions in the medium can screen the surface charge of particles, reducing the zeta potential.
- Particle size: Smaller particles often exhibit higher zeta potentials due to their increased surface area to volume ratio.
PZC Fundamentals
Definition and Relevance
The Point of Zero Charge (PZC) is the pH at which a particle’s surface has no net electrical charge. PZC is a vital concept in surface chemistry, impacting how materials adsorb ions and interact with their environment. This property is crucial for processes like catalysis and environmental remediation, where the surface charge can influence the adsorption of contaminants or reactants.
Measurement Approaches
PZC is typically measured by adjusting the pH of a particle suspension and observing the charge balance at the surface:
- Titration methods: Involve gradually changing the pH and measuring the electrostatic charge until the surface shows no net charge.
- pH drift method: The initial pH is set, and changes are monitored over time to find the balance point where no further pH change occurs.
These methods help in accurately pinpointing the PZC to tailor materials for specific applications.
Factors Affecting PZC
The PZC of materials can be influenced by:
- Chemical composition: Different elements or compounds can shift PZC due to their intrinsic properties.
- Surface structure: Roughness and heterogeneity can affect how ions are adsorbed, altering the PZC.
- Environmental conditions: Temperature and medium composition can shift PZC values.
Key Differences
Electrical Characteristics
While both zeta potential and PZC deal with surface charges, they describe different electrical properties. Zeta potential measures the potential difference across the particle dispersion, whereas PZC identifies the pH at which the surface charge is neutral.
Measurement Context
The methods used to determine zeta potential and PZC also differ significantly. Zeta potential is often measured under dynamic conditions with particles in suspension, reflecting their stability in that state. In contrast, PZC measurement typically involves a static environment where the pH is varied to find the balance point.
Practical Implications
Understanding the distinction between zeta potential and PZC can significantly impact various applications. For instance, in water treatment, knowing the zeta potential helps in designing processes for particle stabilization, while understanding PZC can optimize conditions for maximum contaminant removal. Similarly, in pharmaceuticals, zeta potential can influence the formulation of stable colloidal drug carriers, whereas PZC could affect the drug’s interaction with body tissues.
Applications in Industry
Water Treatment
Water treatment processes greatly benefit from the understanding and application of zeta potential and point of zero charge (PZC). These properties are crucial in optimizing the coagulation and flocculation stages, which are essential for removing contaminants from water.
- Coagulation: Adjusting the pH to near the PZC of suspended particles reduces their charge, promoting aggregation. This process is enhanced by knowing the zeta potential, which helps in selecting the appropriate coagulants to neutralize charges efficiently.
- Flocculation: Once particles form larger aggregates (flocs), the zeta potential helps in stabilizing these flocs, preventing them from breaking down during the treatment process.
This knowledge is applied in both municipal and industrial water treatment facilities to improve clarity, reduce chemical doses, and lower operating costs.
Pharmaceutical Formulations
In pharmaceuticals, the role of zeta potential is critical in the development and stability of formulations, especially for colloidal drug delivery systems. The stability of these colloids is paramount as it affects both the shelf-life and efficacy of the medication.
- Emulsions and Suspensions: Zeta potential measurements ensure that the droplets or particles in these formulations remain stable, preventing separation and sedimentation.
- Targeted Drug Delivery: By manipulating the surface charge measured through zeta potential, scientists can design drug particles that preferentially bind to specific tissues or cells, enhancing the therapeutic efficacy of the drugs.
The use of PZC, while less direct, influences the choice of excipients and the design of drug formulations to optimize their interaction with the body’s pH-sensitive environments.
Material Science
The understanding of zeta potential and PZC is also transformative in the field of material science, particularly in the synthesis and application of nanomaterials and composites.
- Nanotechnology: Zeta potential is used to predict and control the aggregation behavior of nanoparticles, which is crucial for applications ranging from electronics to coatings.
- Ceramics and Composites: Knowledge of PZC allows for the fine-tuning of surface properties to enhance the bonding strength and durability of materials used in a variety of structural applications.
These insights help scientists and engineers create more efficient and longer-lasting materials, impacting industries such as aerospace, automotive, and construction.
Case Studies
Example in Water Purification
A practical application of zeta potential in water purification can be observed in a municipal water treatment facility. The facility faced challenges with high levels of suspended solids and microbial contamination. By implementing a zeta potential-based monitoring system, the facility was able to:
- Adjust the dosage of coagulants accurately, leading to improved removal of turbidity and pathogens.
- Reduce costs associated with overuse of chemicals while also minimizing the environmental impact of the treatment process.
- Achieve consistent water quality that meets regulatory standards and consumer expectations.
This case study highlights how critical zeta potential is in optimizing and troubleshooting water treatment processes.
Example in Drug Delivery Systems
In the pharmaceutical industry, a notable case study involves the development of a targeted drug delivery system using zeta potential measurements. A novel cancer treatment was formulated using nanoparticles designed to bind selectively to cancer cells. Through precise manipulation of the zeta potential:
- The nanoparticles were engineered to have a surface charge optimal for attachment to negatively charged cancer cell membranes.
- This targeting mechanism significantly increased the drug’s efficacy while reducing side effects, as the drug was delivered directly to the tumor site, sparing healthy tissues.
- Clinical trials showed improved patient outcomes and reduced recurrence rates.
Frequently Asked Questions
What is Zeta Potential?
Zeta potential is a scientific term describing the potential difference between the dispersion medium and the stationary layer of fluid attached to the dispersed particle. It serves as a key indicator of the stability of colloidal systems, where higher absolute values suggest greater repulsion among particles, thus indicating stability.
How is PZC Measured?
The point of zero charge (PZC) is measured by monitoring the change in the surface charge of a material as the pH of its surrounding medium is adjusted. This measurement is crucial for predicting how materials will interact with their environment, especially in processes involving adsorption and catalysis.
Why are Zeta Potential and PZC Important?
Understanding zeta potential and PZC is vital for applications such as wastewater treatment, where charge properties affect contaminant removal, and in pharmaceuticals, where these properties influence drug delivery efficiency. They help optimize conditions for product stability and efficacy.
Can Zeta Potential Predict PZC?
While both zeta potential and PZC provide insights into the electrical properties of surfaces, they are measured differently and relate to different aspects of particle behavior in a medium. Thus, zeta potential cannot directly predict PZC, but it can offer complementary information.
Conclusion
The exploration of zeta potential and point of zero charge provides essential insights into the interaction forces at play on material surfaces, crucial for optimizing a range of industrial and scientific processes. By understanding these properties, professionals can better manipulate material behaviors to achieve desired outcomes, enhancing product performance and environmental sustainability.
The study of these electrokinetic properties not only advances scientific understanding but also drives innovation in fields as diverse as healthcare, environmental science, and materials engineering. As such, the distinction between zeta potential and PZC is more than an academic exercise; it is a fundamental part of modern scientific and industrial operations.