Organic chemistry constantly evolves with new methodologies that significantly impact molecular construction and synthesis. Among these, the Wittig Reaction and Wittig Rearrangement stand out as pivotal transformations. Originating from distinct origins and developed by different chemists, these reactions offer diverse pathways for creating complex molecular structures. Each serves unique functions in the synthesis of various organic compounds, showcasing the depth and versatility of organic chemistry.
The Wittig Reaction facilitates the formation of alkenes from ketones or aldehydes using a ylide, whereas the Wittig Rearrangement involves the rearrangement of ethers into alkenes through a reaction that can be either thermally or photochemically driven. These processes are foundational in creating bonds crucial for building complex molecules, differing primarily in their approach and the structures they manipulate.
Focusing on their chemical mechanisms, the Wittig Reaction employs a phosphonium ylide to form a double bond by coupling with a carbonyl group. In contrast, the Wittig Rearrangement shifts an alkoxy group to an adjacent carbon atom, resulting in a different type of alkene. Both reactions have carved niches in synthetic organic chemistry, offering robust tools for constructing varied molecular architectures essential in medicinal chemistry and material science.

Basic Concepts
Wittig Reaction
Definition and Discovery
The Wittig Reaction is a renowned chemical transformation used to synthesize alkenes from carbonyl compounds through the reaction with phosphonium ylides. This reaction was discovered by Georg Wittig, a German chemist, in 1953 and later earned him a Nobel Prize in Chemistry in 1979. The discovery revolutionized synthetic organic chemistry, offering a reliable method for forming double bonds in a controlled manner.
Key Components
The key components of the Wittig Reaction include:
- Phosphonium ylide: A compound containing phosphorus bonded to carbon with a positive charge on phosphorus and a negative charge on carbon.
- Carbonyl compound: Typically a ketone or aldehyde that reacts with the ylide.
- Base: Used to deprotonate the phosphonium salt to generate the ylide.
Wittig Rearrangement
Definition and Discovery
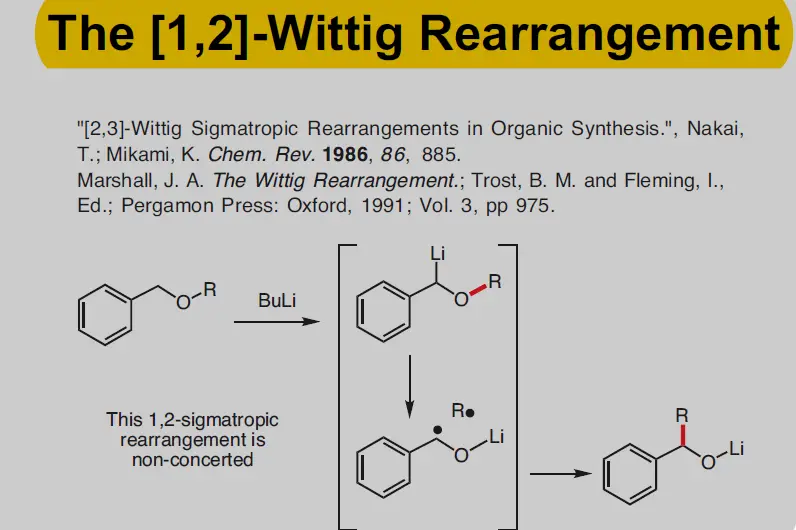
The Wittig Rearrangement is another significant chemical reaction but less commonly discussed compared to its alkene-forming counterpart. It involves the rearrangement of ethers into alkenes under specific conditions. Discovered independently from the Wittig Reaction, this rearrangement expands the toolkit of chemists, particularly in the synthesis of complex molecular structures.
Key Components
The primary components involved in the Wittig Rearrangement include:
- Ether: The starting material which undergoes rearrangement.
- Heat or light: Depending on the reaction pathway, either thermal energy or UV light is required to initiate the rearrangement.
Chemical Mechanisms
Wittig Reaction Mechanism
Step-by-step Process
The mechanism of the Wittig Reaction can be described as follows:
- Generation of Ylide: The phosphonium salt is treated with a strong base to form the ylide.
- Addition to Carbonyl: The ylide adds to the carbonyl carbon of the ketone or aldehyde.
- Formation of Oxaphosphetane: A four-membered ring intermediate, oxaphosphetane, is formed.
- Cleavage: The oxaphosphetane ring opens to form the alkene and a phosphine oxide.
Role of Phosphonium Ylide
Phosphonium ylide plays a crucial role in the Wittig Reaction as the agent that directly forms the new carbon-carbon double bond with the carbonyl carbon, effectively altering the molecular structure.
Wittig Rearrangement Mechanism
Step-by-step Process
The Wittig Rearrangement follows these steps:
- Formation of Alkoxyallyl Cation: Under thermal conditions, the ether forms an alkoxyallyl cation.
- Rearrangement: The alkoxy group migrates to the adjacent carbon, forming a new alkene.
Thermal vs. Photochemical Pathways
- Thermal Pathway: Involves the application of heat, typically leading to a straightforward migration and formation of the product.
- Photochemical Pathway: Uses UV light to initiate the rearrangement, potentially offering more control and selectivity in the reaction.
Applications
In Synthesis
Synthetic Applications of Wittig Reaction
The Wittig Reaction is extensively used in the synthesis of complex molecules such as vitamins, steroids, and pheromones. Its ability to form alkenes precisely makes it indispensable in the construction of large, complex organic molecules.
Synthetic Applications of Wittig Rearrangement
Though less common, the Wittig Rearrangement finds its use in the synthesis of specialized organic compounds where rearrangements are more advantageous than direct substitutions.
Industrial Impact
Commercial Uses
Both reactions have applications beyond laboratory scales, influencing the production of materials like plastics, fragrances, and even pharmaceuticals.
Influence on Drug Development
The Wittig Reaction, in particular, has had a profound impact on drug development, enabling the synthesis of prostaglandins, steroids, and various other bioactive molecules crucial for medical treatments.
Comparative Analysis
Reaction Conditions
Solvents and Temperature
The Wittig Reaction typically utilizes polar aprotic solvents such as THF (tetrahydrofuran) or DMF (dimethylformamide), which effectively dissolve phosphonium ylides and promote the nucleophilic attack on the carbonyl carbon. Optimal temperatures for this reaction are generally in the range of 0°C to 25°C, helping to control the rate and favor the formation of desired geometric isomers of alkenes.
In contrast, the Wittig Rearrangement often requires high temperatures or specific light conditions depending on the chosen pathway. The reaction can proceed in a variety of solvents, with preferences sometimes dictated by the stability of the intermediate cations formed during the rearrangement process.
Catalysts and Reagents
Strong bases like n-butyllithium or sodium amide are crucial for the Wittig Reaction to deprotonate the phosphonium salt to generate the ylide. No additional catalysts are typically needed, as the ylide itself is reactive enough.
For the Wittig Rearrangement, acidic or basic catalysts can be employed to initiate the migration of the alkoxy group. The choice of catalyst often depends on the specific substrate and desired pathway (thermal vs. photochemical).
Reaction Outcomes
Types of Products Formed
The Wittig Reaction exclusively produces alkenes, with the stereochemistry of the product being a notable variable. Depending on the substituents attached to the reacting carbonyl compound and the ylide, the outcome can be either a cis or trans alkene.
The Wittig Rearrangement yields alkenes as well, but the positioning of the double bond and the structure of the side chains can vary significantly based on the original ether’s structure and the reaction conditions.
Yield and Efficiency
The Witt
ig Reaction is highly efficient, often resulting in high yields of the desired alkene product, especially when stabilizing groups are present to facilitate the reaction. The efficiency can be influenced by the precise control of reaction parameters and the purity of the reagents.
The Wittig Rearrangement’s yield can vary, largely depending on the stability of the intermediate and the control over the reaction conditions. Yields might be lower compared to the Wittig Reaction, especially in cases where the rearrangement pathway is less straightforward or the intermediate is particularly unstable.
Advantages and Limitations
Wittig Reaction
Benefits in Organic Synthesis
The Wittig Reaction offers several advantages:
- High selectivity for the formation of alkenes, allowing for precise control over the product’s geometry.
- Versatility in reacting with a wide range of carbonyl compounds.
- Functional group tolerance, making it compatible with various sensitive molecular structures.
Limitations and Challenges
Despite its benefits, the Wittig Reaction has limitations:
- Steric hindrance can significantly affect the reaction, particularly with bulky substituents.
- Sensitivity to moisture and air, requiring careful handling of reagents.
- The cost and availability of phosphorus reagents can also be a drawback in larger-scale applications.
Wittig Rearrangement
Unique Advantages
The Wittig Rearrangement is particularly useful for:
- Structural diversity in the synthesis of complex molecules, as it can introduce new functional groups and alter molecular scaffolding.
- Mild reaction conditions for the photochemical pathway, which can preserve sensitive functionalities within the molecule.
Limitations and Challenges
However, the Wittig Rearrangement faces its own challenges:
- Control over product distribution can be difficult, especially when multiple rearrangement pathways are possible.
- Requirement for specific conditions such as high temperature or UV light, which may not be suitable for all substrates.
Recent Developments
Innovations in Wittig Reaction
Recent advancements in the Wittig Reaction focus on:
- Developing more stable ylide reagents that are easier to handle and store.
- Exploring asymmetric versions of the reaction to create chiral alkenes, enhancing its utility in the synthesis of biologically active compounds.
- Environmental considerations, such as the development of greener reaction conditions and the use of less toxic reagents.
Innovations in Wittig Rearrangement
In the realm of Wittig Rearrangement, innovations include:
- Photochemical enhancements that allow for greater control over the reaction pathway and reduce the need for high temperatures.
- Catalytic methods that increase the efficiency and selectivity of the rearrangement, broadening its applicability in organic synthesis.
Future Trends
Looking forward, both reactions are likely to see continued refinement. There is a strong trend towards:
- Sustainability, with research aimed at making these reactions more environmentally friendly.
- Automation and high-throughput experimentation to rapidly screen conditions and reagents for optimal outcomes.
- Integration into complex synthetic sequences, particularly in the pharmaceutical industry where these methods can streamline the production of active pharmaceutical ingredients.
Frequently Asked Questions
What is Wittig Reaction?
The Wittig Reaction is a chemical process used in organic chemistry to synthesize alkenes from carbonyl compounds like ketones or aldehydes through the reaction with phosphonium ylides. It is highly valued for its ability to form double bonds with precision, which is critical in the synthesis of complex molecules.
What is Wittig Rearrangement?
Wittig Rearrangement is a lesser-known but equally significant reaction where ethers are converted into alkenes through a rearrangement process. This can occur under thermal conditions or through photochemical means, depending on the specific requirements of the synthesis.
How do Wittig Reaction and Wittig Rearrangement differ?
While both the Wittig Reaction and Wittig Rearrangement are used to create alkenes, they differ in their mechanisms and the starting materials used. The Wittig Reaction uses phosphonium ylides and carbonyl compounds to form a double bond directly, whereas the Wittig Rearrangement involves the migration of an alkoxy group to form a new alkene.
Why are these reactions important in organic synthesis?
Both reactions are fundamental in organic synthesis due to their ability to construct alkenes, a common functionality in organic compounds. Their utility extends to pharmaceuticals, agrochemicals, and materials science, where precise molecular construction is crucial.
Conclusion
The Wittig Reaction and Wittig Rearrangement are cornerstone methodologies in the field of organic chemistry. Each offers a unique approach to alkene synthesis, underpinning many advanced synthetic strategies used in creating complex molecular frameworks. Their continued relevance in academic and industrial settings underscores the dynamic nature of chemical synthesis and the ongoing need for efficient, versatile reaction processes.
As research progresses, both reactions are likely to see refinements and expanded applications, particularly in the synthesis of novel organic compounds with significant biological or physical properties. Understanding and utilizing these reactions will remain a critical skill set for chemists aiming to push the boundaries of what can be achieved through synthetic organic chemistry.

