In the realm of organic chemistry, the Wittig and Wittig Horner reactions stand as pivotal methodologies for synthesizing alkenes from carbonyl compounds. These reactions not only epitomize the ingenuity of chemical synthesis but also highlight the diversity of approaches chemists can employ to achieve similar outcomes. Each reaction brings its unique set of reactants, conditions, and mechanisms to the table, offering a rich tapestry of chemical knowledge.
The Wittig reaction utilizes phosphorus ylides to convert carbonyl compounds into alkenes, whereas the Wittig Horner reaction, also known as the Horner-Wadsworth-Emmons reaction, employs phosphonate esters for the same purpose. Despite their common goal, the differences in their reactants, mechanisms, and outcomes underscore the versatility and adaptability of organic synthesis strategies.
Focusing on these two reactions provides a window into the broader world of synthetic organic chemistry, revealing the nuanced choices that chemists make based on the specific needs of their synthesis. From the intricacies of their mechanisms to the subtleties of their stereochemical outcomes, the Wittig and Wittig Horner reactions illuminate the art and science of chemical synthesis, demonstrating how different tools and strategies can be applied to construct complex molecular architectures.
Core Chemistry
Wittig Reaction Overview
The Wittig reaction represents a cornerstone of organic chemistry, facilitating the synthesis of alkenes from carbonyl compounds. Unveiled by Georg Wittig, a Nobel Laureate, this reaction has revolutionized approaches to molecular construction, offering a versatile pathway to carbon-carbon double bonds.
At its core, the Wittig reaction involves the interaction between a carbonyl compound and a phosphorus ylide, leading to the formation of an alkene. This process bypasses the need for more cumbersome and less efficient methods of alkene synthesis, showcasing its elegance and efficiency.
Wittig Horner Reaction Explained
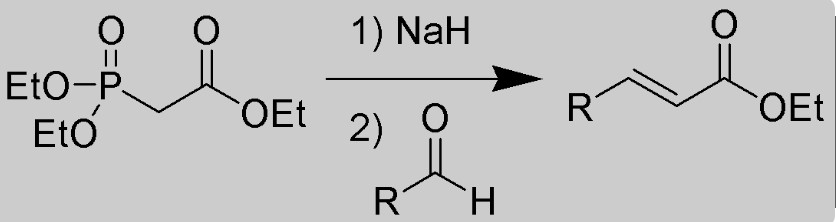
Parallel to the Wittig reaction, the Wittig Horner reaction, also known as the Horner-Wadsworth-Emmons (HWE) reaction, stands as another pivotal method for alkene synthesis. This variant introduces phosphonate esters into the mix, broadening the scope of carbonyl compounds that can be efficiently converted into alkenes.
Developed through the collaborative efforts of chemists Horner, Wadsworth, and Emmons, the Wittig Horner reaction expands the toolkit available to chemists, offering nuanced control over the stereochemistry of the resulting alkenes—a critical aspect in the synthesis of complex organic molecules.
Key Components
Phosphorus Ylides in Wittig
Phosphorus ylides are the linchpin of the Wittig reaction. These compounds feature a unique phosphorus-carbon bond that is both polar and ready to engage in chemical reactions. Preparing phosphorus ylides typically involves the treatment of a phosphonium salt with a strong base, setting the stage for their role in alkene synthesis.
In the Wittig reaction, phosphorus ylides attack the carbonyl carbon of the substrate, initiating a sequence of steps that culminate in the formation of a new carbon-carbon double bond. The versatility and reactivity of phosphorus ylides make them invaluable tools in the chemist’s arsenal, enabling the straightforward synthesis of alkenes from a variety of carbonyl compounds.
Phosphonate Esters in Wittig Horner
Phosphonate esters play a central role in the Wittig Horner reaction. These compounds, which can be seen as cousins to phosphorus ylides, offer an alternative route to alkene synthesis. The preparation of phosphonate esters involves the reaction of a phosphonate with a halide under basic conditions, leading to a wide array of esters ready for use in synthesis.
In the reaction mechanism, phosphonate esters provide a means to form alkenes with greater control over the stereochemical outcome, an advantage in the synthesis of complex molecules where the configuration of double bonds is critical. The Wittig Horner reaction, thus, represents a complementary approach to the Wittig reaction, with phosphonate esters enabling a broader scope of carbonyl compound transformations.
Mechanistic Insights
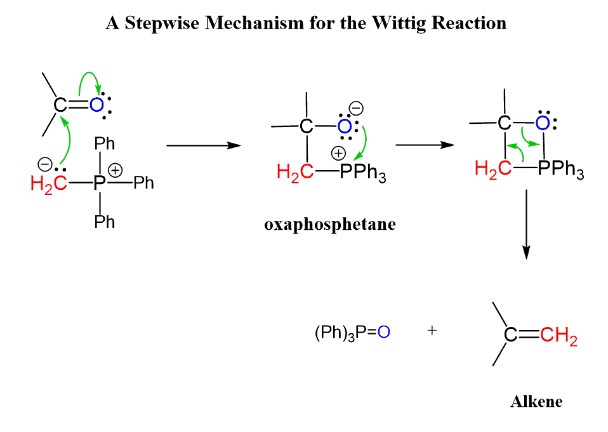
Reaction Mechanism of Wittig
The mechanism of the Wittig reaction unfolds through several key steps, each integral to the formation of the alkene product. Here’s a step-by-step guide to this transformative process:
- Formation of the Ylide: The reaction begins with the generation of the phosphorus ylide from a phosphonium salt.
- Nucleophilic Attack: The ylide then attacks the carbonyl carbon, forming a betaine intermediate.
- Cyclization: This intermediate quickly undergoes cyclization to form an oxaphosphetane.
- Alkene Formation: Finally, the oxaphosphetane collapses, yielding the desired alkene and a phosphine oxide byproduct.
This sequence of steps not only highlights the efficiency of the Wittig reaction in synthesizing alkenes but also underscores the reaction’s versatility, applicable across a range of carbonyl compounds.
Reaction Mechanism of Wittig Horner
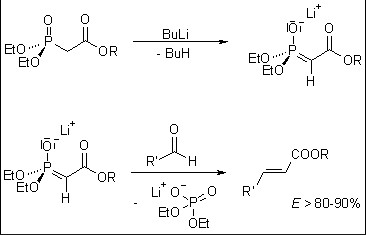
The Wittig Horner reaction mechanism, while sharing a common goal with the Wittig reaction, diverges in its path to alkene formation. Here are the critical steps:
- Phosphonate Ester Activation: Initially, the phosphonate ester is activated by deprotonation, forming a carbanion.
- Attack on the Carbonyl Compound: The carbanion then attacks the carbonyl carbon, leading to the formation of a betaine-like intermediate.
- Alkene Formation: This intermediate then rearranges, directly yielding the alkene and a phosphate byproduct.
This mechanism, distinct from the Wittig reaction, illustrates the nuanced differences between the two approaches, with the Wittig Horner reaction offering enhanced control over stereochemical outcomes and a broader applicability to different carbonyl substrates.
Reactivity and Conditions
Conditions for Wittig
The Wittig reaction’s versatility partly stems from its broad tolerance of various solvents, temperatures, and catalysts, making it a flexible tool for chemists. Here are the typical conditions under which the Wittig reaction is performed:
- Solvents: The choice of solvent can greatly influence the reaction’s outcome. Common solvents include dichloromethane, tetrahydrofuran (THF), and toluene. These solvents are chosen for their ability to dissolve both reactants and products effectively while not interfering with the reaction mechanism.
- Temperature: Wittig reactions can occur at a range of temperatures, from room temperature to reflux conditions. The specific temperature often depends on the reactivity of the ylide and the carbonyl compound involved.
- Catalysts: While the Wittig reaction does not typically require catalysts, the use of Lewis acids can enhance reactivity, especially in cases where the carbonyl compound is less reactive.
These conditions underscore the reaction’s adaptability, allowing chemists to tweak parameters to optimize yields and selectivity based on the specific needs of their synthesis.
Conditions for Wittig Horner
The Wittig Horner reaction, while similar in its goals to the Wittig reaction, operates under a distinct set of conditions that can vary from those of the Wittig:
- Solvents: Polar aprotic solvents, such as dimethylformamide (DMF) and acetonitrile (MeCN), are commonly used. These solvents facilitate the deprotonation of the phosphonate ester and the subsequent reaction steps.
- Temperature: Generally, the Wittig Horner reaction is conducted at temperatures ranging from room temperature to slightly elevated temperatures. The precise temperature can influence the reaction rate and the stereochemistry of the product.
- Base: A strong base is crucial for deprotonating the phosphonate ester to generate the active carbanion. Common bases include sodium hydride (NaH) and potassium tert-butoxide (KOtBu).
The conditions for the Wittig Horner reaction reflect its unique mechanism and the nature of its reactants, with each parameter chosen to maximize efficiency and control over the product’s formation.
Product Outcomes
Alkene Stereochemistry in Wittig
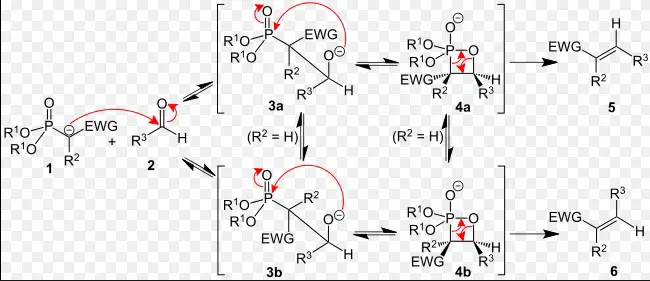
The stereochemistry of alkenes produced by the Wittig reaction is a critical aspect, with the E/Z selectivity being a notable point of interest. Several factors influence this selectivity:
- Ylide Stability: Stabilized ylides tend to produce E-alkenes, while non-stabilized ylides favor Z-alkenes.
- Reaction Conditions: Solvent, temperature, and the presence of additives can affect the stereochemical outcome.
- Substrate Structure: The nature of the carbonyl compound, especially its steric and electronic properties, plays a significant role in determining the E/Z ratio of the product.
These factors highlight the nuanced control chemists can exert over the reaction, tailoring conditions to achieve the desired stereochemical configuration.
Alkene Stereochemistry in Wittig Horner
The Wittig Horner reaction provides enhanced control over alkene stereochemistry, often yielding products with predictable E-selectivity. This control is attributed to the nature of the phosphonate ester and the reaction conditions employed. Factors affecting stereochemistry in the Wittig Horner reaction include:
- Ester Substitution: The substitution pattern on the phosphonate ester can influence the stereochemistry of the alkene, with certain substituents favoring E or Z outcomes.
- Base Strength: The choice of base can affect the carbanion’s reactivity and thus the stereochemistry of the alkene.
- Solvent Effect: The solvent can impact the conformation of intermediates, thereby influencing the E/Z ratio of the product.
Applications and Examples
Synthetic Applications of Wittig
The Wittig reaction has been employed in the synthesis of a wide array of complex molecules, from natural products to materials science applications. Here are a few examples:
- Vitamin A Synthesis: One of the hallmark applications of the Wittig reaction is in the synthesis of Vitamin A, showcasing its ability to construct complex molecules.
- Polyene Synthesis: The Wittig reaction is instrumental in synthesizing polyenes, critical components in many natural products and polymers.
These applications demonstrate the Wittig reaction’s broad utility in organic synthesis, enabling the construction of molecules with diverse functionalities.
Synthetic Applications of Wittig Horner
The Wittig Horner reaction finds its strength in applications requiring strict control over alkene stereochemistry and in the synthesis of molecules with sensitive functional groups. Examples include:
- Steroid Synthesis: The Wittig Horner reaction has been used to synthesize steroids with precise stereochemical configurations.
- Material Science: In materials science, the Wittig Horner reaction aids in the construction of molecular electronics and photonics components, where the geometry of double bonds is critical.
Advantages and Limitations
Pros and Cons of Wittig
Advantages:
- Broad applicability to a wide range of carbonyl compounds.
- High yields and straightforward reaction conditions.
Limitations:
- Sometimes limited control over alkene stereochemistry.
- Generation and handling of phosphorus ylides can be challenging.
Pros and Cons of Wittig Horner
Advantages:
- Enhanced control over alkene stereochemistry.
- Applicable to sensitive substrates due to milder reaction conditions.
Limitations:
- Requires the synthesis of phosphonate esters, which can be an additional step in the reaction preparation.
- May require stronger bases, which can complicate purification processes.
Frequently Asked Questions
What is a Wittig Reaction?
The Wittig reaction is a chemical process used to synthesize alkenes from carbonyl compounds using phosphorus ylides as a key reagent. This reaction is pivotal in organic chemistry for constructing carbon-carbon double bonds, offering a versatile tool for building complex molecular structures from simpler substrates.
How does the Wittig Horner Reaction differ?
The Wittig Horner reaction, or Horner-Wadsworth-Emmons reaction, differs from the Wittig reaction primarily in its use of phosphonate esters instead of phosphorus ylides. This variation impacts the reaction’s mechanism, stereochemical outcomes, and the types of substrates that can be effectively converted into alkenes.
Why are these reactions important in organic synthesis?
Both the Wittig and Wittig Horner reactions are crucial in organic synthesis due to their ability to form carbon-carbon double bonds efficiently. They enable chemists to construct a wide range of complex organic molecules, including natural products, pharmaceuticals, and materials, with high precision and selectivity.
When would you choose one reaction over the other?
The choice between the Wittig and Wittig Horner reactions depends on several factors, including the desired stereochemical outcome, the nature of the starting materials, and the specific conditions under which the reaction is carried out. The Wittig reaction is often preferred for its simplicity and broad applicability, while the Wittig Horner reaction is chosen for its ability to yield more stereoselectively controlled alkenes.
Conclusion
The Wittig and Wittig Horner reactions exemplify the beauty and complexity of organic synthesis, each serving as a testament to the creative strategies chemists employ to build complex molecules from simpler ones. While they share a common goal, the distinctions in their mechanisms, reactants, and outcomes highlight the depth and breadth of synthetic chemistry. As tools in the chemist’s arsenal, these reactions underscore the importance of understanding and selecting the right synthetic pathway to achieve the desired molecular architecture.
By dissecting the nuances of these reactions, we not only appreciate the ingenuity behind their development but also recognize the pivotal role they play in advancing the field of organic chemistry. Whether forging new paths in drug discovery or paving the way for new materials, the Wittig and Wittig Horner reactions continue to fuel innovation and discovery, embodying the endless quest for knowledge and mastery over the molecular fabric of our world.