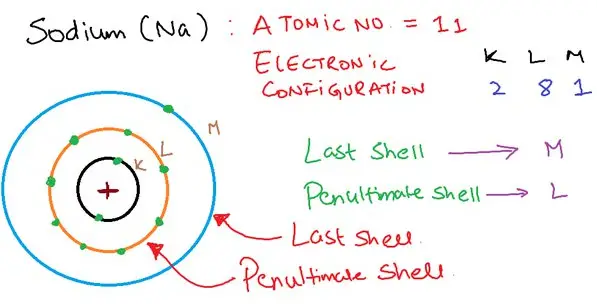
Electrons orbit the nucleus of an atom in various energy levels known as shells. Among these, the valence shell and penultimate shell play critical roles in determining the chemical properties of an element. The differences between these shells are not merely academic; they are fundamental to the practical application of chemistry in fields ranging from materials science to biochemistry.
The valence shell is the outermost shell of an atom and contains the electrons most involved in chemical reactions. It largely determines an element’s reactivity and its ability to form bonds with other atoms. In contrast, the penultimate shell is the second to last shell, which can also play a significant role in the chemical behavior of an atom, particularly in elements with many electron shells.
A deeper understanding of these shells enhances our ability to predict and manipulate the chemical behavior of elements. For instance, knowing that the valence electrons define an element’s bonding capacity while the electrons in the penultimate shell can influence its ionization energy and oxidation states, provides valuable insights into the nature of atoms.
Basic Concepts
Atomic Structure Overview
Definition of an Atom
An atom is the smallest unit of ordinary matter that forms a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are the basic building blocks of chemistry.
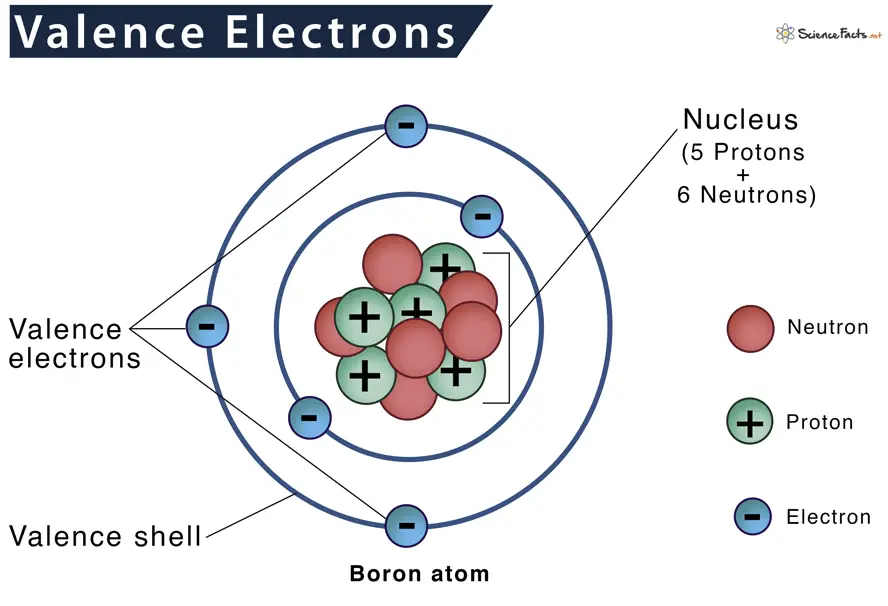
Key Components: Protons, Neutrons, Electrons
Atoms consist of three types of particles:
- Protons: Positively charged particles found in the nucleus.
- Neutrons: Neutral particles that also reside in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus.
The number of protons in the nucleus defines the chemical element of the atom, while the number of electrons affects its reactivity and electrical properties.
Electron Shells
Explanation of Electron Shells
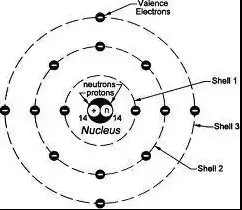
Electron shells are the paths electrons take around an atom’s nucleus. These shells represent different energy levels. The shell closest to the nucleus has the lowest energy, and the energy increases with each subsequent shell.
How Electrons are Organized in Shells
Electrons fill shells in a manner that minimizes the energy of the atom. The first shell can hold up to 2 electrons, the second up to 8, and the third can hold up to 18:
- First shell (closest): Up to 2 electrons
- Second shell: Up to 8 electrons
- Third shell: Up to 18 electrons
The distribution is dictated by quantum mechanics, specifically the Pauli exclusion principle and Hund’s rule.
Valence Shell
Definition and Role
The valence shell is the outermost shell of an electron within an atom. It plays a critical role in determining an atom’s chemical properties by providing the electrons that participate in chemical bonding.
Importance in Chemical Bonding and Reactions
Electrons in the valence shell are the ones that interact with other atoms to form chemical bonds. This interaction can include sharing, gaining, or losing electrons to form stable electronic configurations.
Characteristics of Valence Shells
Electron Configuration
The configuration of electrons in the valence shell determines how an element reacts chemically. For example, helium (He) has a full valence shell with two electrons, making it inert.
Examples from the Periodic Table
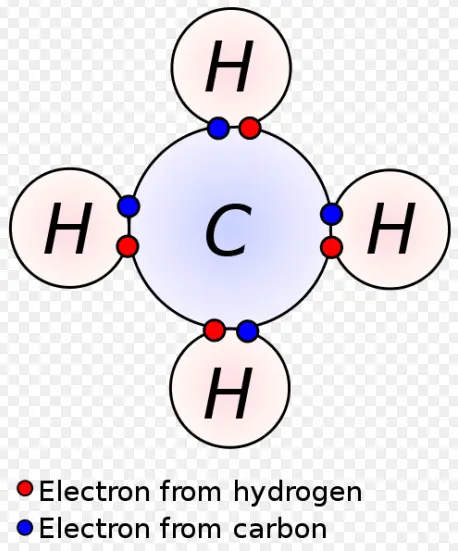
- Hydrogen (H): 1 electron in its valence shell, highly reactive.
- Carbon (C): 4 electrons in its valence shell, forms four bonds.
Penultimate Shell
Definition and Significance
The penultimate shell is the second to the last electron shell in an atom. It becomes especially relevant in atoms with many electron shells.
Role in Atom’s Stability and Reactivity
The penultimate shell can influence the atom’s stability and its chemical reactivity. For elements in higher periods of the periodic table, the penultimate shell may contain more electrons than the valence shell, affecting the atom’s properties.
Characteristics of Penultimate Shells
Electron Capacity and Common Configurations
The penultimate shell typically follows the same filling rules as other shells but is often not completely filled in lighter elements. For heavier elements, it can be closer to capacity:
- Oxygen (O): 2 electrons in the penultimate shell.
- Chlorine (Cl): 8 electrons in the penultimate shell, contributing to its high reactivity.
Influence on Atom’s Chemical Properties
The electron configuration of the penultimate shell can affect the energy levels of the valence electrons, thereby impacting the atom’s ionization energy and oxidation states. This is crucial for understanding the chemistry of transition metals and other complex elements.
Comparing Valence and Penultimate Shells
Key Differences
Electron Involvement in Chemical Reactions
The valence shell electrons are directly involved in chemical bonding. These electrons are the most accessible for chemical reactions, as they are the least bound to the nucleus. For instance, in a simple reaction like sodium (Na) bonding with chlorine (Cl) to form sodium chloride (NaCl), it is the valence electron of sodium that is transferred to the valence shell of chlorine.
Conversely, electrons in the penultimate shell generally play a more indirect role. They influence the energy and distribution of the valence electrons, which can alter reactivity under certain conditions but are not usually participants in bond formation themselves.
Stability Contributions to the Atom
The stability of an atom can often depend on both its valence and penultimate shells. While the full or nearly full valence shell contributes to chemical inertness, as seen in noble gases, the penultimate shell can provide stability through its effect on electron shielding and the overall energy state of the atom.
Similarities
Integral Part of Atomic Structure
Both the valence and penultimate shells are crucial components of an atom’s structure. They are essential for determining the chemical identity and properties of the element.
Influence on Atom’s Overall Behavior
Despite their different roles, both shells significantly influence an atom’s overall behavior, including its reactivity, bonding capacity, and interactions with electromagnetic fields.
Impact on Chemical Properties
Reactivity
How Valence and Penultimate Shells Affect Reactivity
The reactivity of an atom is primarily determined by its valence electrons. However, the electrons in the penultimate shell can also have a substantial impact, especially in elements with larger atomic numbers. These inner electrons can shield the valence electrons from the nuclear charge, making them more available for bonding and thus altering the atom’s reactivity.
Examples in Common Elements
- Fluorine (F): Highly reactive due to its seven valence electrons, which eagerly gain an electron to fill the valence shell.
- Lead (Pb): Shows variable reactivity due to its complex electron configuration involving both the valence and penultimate shells.
Bonding Capabilities
Differences in Bonding Due to Shell Characteristics
The capacity of an atom to form chemical bonds varies based on the configuration of both its valence and penultimate shells. Elements with a nearly full or full penultimate shell, such as transition metals, exhibit varied bonding behaviors due to the involvement of d-orbitals which lie in the penultimate shell.
Impact on Molecule Formation
The characteristics of these shells influence how molecules form and the types of bonds an atom can make. For example, carbon can form four single covalent bonds in methane, whereas oxygen typically forms two due to the different configurations of their valence shells.
Advanced Considerations
Exceptions and Anomalies
Transition Metals and Lanthanides
Transition metals and lanthanides often have unusual electron configurations where the penultimate shell plays a significant role in bonding and chemical properties. Their d and f orbitals, respectively, can be partially filled and actively involved in bonding, leading to complex chemistry and multiple oxidation states.
Unusual Electron Configurations and Their Effects
Examples include chromium and copper, which have electron configurations that differ from what simple models predict. These anomalies affect their chemical behavior and the types of compounds they can form.
Theoretical Implications
Quantum Mechanical Perspective on Shells
Quantum mechanics provides a deeper understanding of how electron shells are structured and behave. The principles of quantum mechanics explain the shapes and energies of atomic orbitals, which in turn influence how shells are filled and how atoms interact.
Research and Discoveries Shaping Understanding
Ongoing research into the quantum mechanical properties of electron shells continues to refine our understanding of chemical bonding and atomic interactions. Recent studies in areas like superconductivity and nanotechnology frequently revisit the role of valence and penultimate shells, providing new insights into their influence on material properties.
Frequently Asked Questions
What is a Valence Shell?
The valence shell is the outermost electron shell of an atom. This shell holds the electrons that participate most actively in chemical reactions and bonding with other atoms. The configuration of electrons in this shell determines many of an element’s chemical properties.
What is a Penultimate Shell?
The penultimate shell is the second to last electron shell in an atom. It plays a secondary, yet vital role in an atom’s chemical properties by influencing the behavior of the valence electrons, especially in atoms with a larger number of shells.
How do Electron Shells Affect Reactivity?
Electron shells, particularly the valence shell, dictate an atom’s reactivity. Electrons in the valence shell are the ones lost, gained, or shared during chemical reactions, determining how an element interacts with others. The penultimate shell can also impact reactivity by affecting valence shell electron distribution.
Can Penultimate Shells Participate in Bonding?
While penultimate shells generally do not participate directly in bonding, their electron configuration can influence the energy levels and distribution of the valence electrons, thereby indirectly affecting bonding capabilities and the stability of bonds formed.
Conclusion
Exploring the differences between the valence and penultimate shells not only enriches our understanding of chemical principles but also enhances our ability to predict and manipulate the behavior of elements. By appreciating how these shells contribute to an atom’s properties, scientists and educators can develop more effective strategies for teaching and applying chemistry in real-world scenarios.
The knowledge of these electron shells, therefore, is not just academic; it is a cornerstone of modern chemical science, influencing everything from innovative materials development to pharmaceutical research. As we continue to uncover the subtleties of atomic behavior, the significance of both the valence and penultimate shells remains at the forefront of scientific inquiry.