Transuranic elements and radioisotopes are terms that are often used interchangeably, but they actually refer to two different types of materials. In this blog post, we will discuss the differences between these two types of elements and explain why it is important to understand the difference between them.
Characteristics of transuranic elements
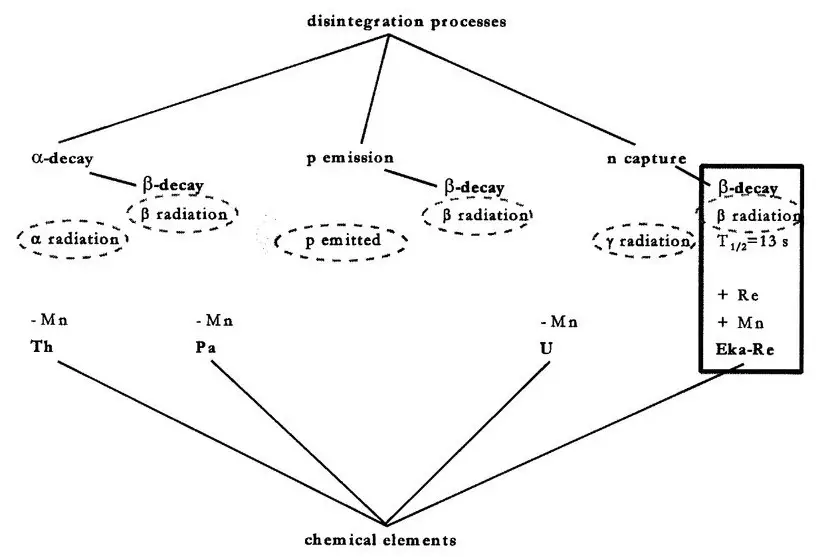
Transuranic elements, also known as transuranium elements, are elements with atomic numbers greater than 92, which is the atomic number of uranium. These elements are very different from radioisotopes, which are unstable atoms that have an excess of neutrons.
Transuranic elements are heavier and more stable than radioisotopes, and they are not found naturally on Earth. Instead, they must be synthesized in a laboratory environment. Transuranic elements tend to have longer half-lives than radioisotopes, which makes them more suitable for certain applications.
Transuranic elements are also more expensive to produce than radioisotopes, and they require special handling and disposal procedures due to their radiological hazards.
Characteristics of radioisotopes

Radioisotopes are atoms with an unstable nucleus that emit radiation. They are used in a variety of applications, from medical imaging to radioactive dating. Transuranic elements, on the other hand, are atoms with an atomic number greater than 92, which means they have more protons in their nucleus than uranium.
Transuranic elements, on the other hand, are atoms with an atomic number greater than 92, which means they have more protons in their nucleus than uranium. While both are radioactive, there is an important distinction between the two. Radioisotopes are unstable and emit radiation, while transuranic elements are generally stable and do not emit radiation.
Radioisotopes are also used in a wide variety of applications, from medical imaging to radioactive dating, while transuranic elements are primarily used in nuclear reactors and weapons. Additionally, radioisotopes have a much shorter half-life than transuranic elements, making them safer and easier to store.
Differences between transuranic elements and radioisotopes
Transuranic elements and radioisotopes are both radioactive substances, but they have some key differences that set them apart. Transuranic elements have an atomic number greater than 92, meaning they have more than 92 protons. Radioisotopes, on the other hand, are unstable forms of elements that have the same number of protons but different numbers of neutrons.
Radioisotopes decay over time, while transuranic elements are more stable and don’t decay. Transuranic elements also have longer half-lives than radioisotopes, so they take longer to decay.
Both types of substances have important uses in nuclear energy, medicine, and research, but understanding the differences between them is essential for proper use.
Applications of transuranic elements
Transuranic elements are a class of elements found beyond uranium in the periodic table. These elements include such elements as plutonium, americium, curium, and californium. In contrast to radioisotopes, transuranic elements are not naturally occurring in nature.
Instead, they are created through processes such as particle bombardment and nuclear fission. Applications of transuranic elements include nuclear power generation, medical imaging, radiation therapy, and radiometric dating.
In nuclear power generation, transuranic elements are used to fuel the nuclear reaction, while in medical imaging, they are used to create images of internal organs. Radiation therapy uses transuranic elements to treat cancer, while radiometric dating is used to calculate the age of rocks and other materials.
Applications of radioisotopes
Radioisotopes are atoms that have an unstable nucleus, leading them to decay and emit radiation. Transuranic elements are elements with atomic numbers higher than uranium which are created when radioactive isotopes of uranium and thorium undergo nuclear fission. The main difference between radioisotopes and transuranic elements is that radioisotopes are naturally occurring and transuranic elements are artificially created.
The main difference between radioisotopes and transuranic elements is that radioisotopes are naturally occurring and transuranic elements are artificially created. Radioisotopes are used in a variety of applications across a number of industries, such as medical imaging, nuclear power generation, and materials testing. On the other hand, transuranic elements are used mainly in nuclear reactors as a fuel source.
Bottom Line
In conclusion, the main difference between transuranic elements and radioisotopes is that transuranic elements are created by nuclear fusion, while radioisotopes are created by radioactive decay. Transuranic elements are elements with atomic numbers higher than 92, while radioisotopes are any isotope of an element that has an unstable nucleus. Both transuranic elements and radioisotopes have various uses in research, industry, and medicine.
Both transuranic elements and radioisotopes have various uses in research, industry, and medicine.
