Chemical nomenclature often brings to light compounds with names that are as intriguing as their properties and applications. Among these, thiocyanate and isothiocyanate stand out for their unique characteristics and the pivotal roles they play in various scientific and industrial fields. Despite the similarity in their names, these compounds have distinct structures, properties, and uses that set them apart.
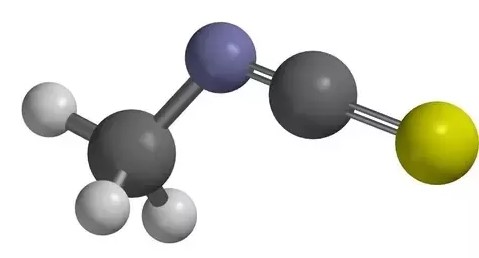
Thiocyanate and isothiocyanate are different in their chemical structure and reactivity. Thiocyanate (SCN-) consists of sulfur, carbon, and nitrogen atoms aligned linearly, whereas isothiocyanate (NCS-) has a similar composition but with a different arrangement of atoms. This structural variation leads to differences in their physical and chemical properties, influencing how each compound interacts in biological systems and chemical reactions.
The significance of these compounds extends across a broad spectrum of applications, from their role in plant defense mechanisms as part of natural compounds found in cruciferous vegetables to their use in synthetic chemistry. Thiocyanates are often utilized in pharmaceuticals and as ligands in coordination chemistry, while isothiocyanates are notable for their anticancer properties and use in agricultural biopesticides. Understanding the differences and similarities between these compounds not only enriches chemical knowledge but also opens up avenues for research and innovation in multiple disciplines.
Thiocyanate Basics
Definition and Structure
Thiocyanate (SCN-) is a chemical compound consisting of sulfur (S), carbon (C), and nitrogen (N) atoms. This compound is known for its linear structure, with the sulfur atom connected to the carbon atom, which in turn is bonded to the nitrogen atom. This linear arrangement is critical for its reactivity and interaction with other chemicals.
Common Sources
Thiocyanates are found in various natural and synthetic sources. Naturally, they are produced in the body from the ingestion of certain foods containing glucosinolates, found in cruciferous vegetables like broccoli and cabbage. Industrially, thiocyanates are synthesized through the reaction of cyanide with elemental sulfur or thiosulfate salts. These processes highlight the compound’s versatility and widespread availability.
Applications
The applications of thiocyanates are diverse, reflecting their broad utility in different fields:
- Pharmaceuticals: Used as building blocks in drug synthesis.
- Agriculture: Serve as fertilizers and herbicides.
- Chemical Synthesis: Important reagents in the production of various organic compounds.
- Photography: Utilized in traditional photographic processes for toning.
Isothiocyanate Basics
Definition and Structure
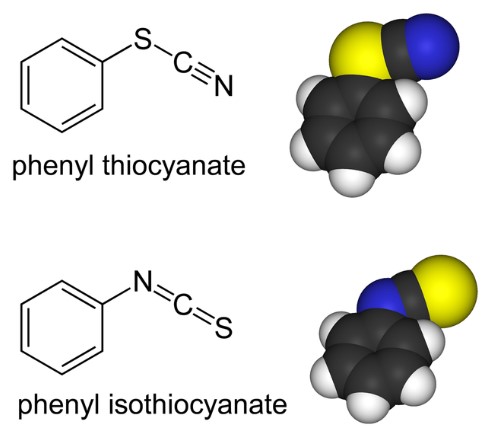
Isothiocyanate (NCS-) is another compound that features sulfur, carbon, and nitrogen. However, its structure is distinct from thiocyanate; the nitrogen atom is bonded directly to the carbon, which is then connected to the sulfur atom. This configuration affects its physical and chemical properties significantly.
Natural Occurrence
Isothiocyanates are primarily known for their presence in cruciferous vegetables, such as broccoli, brussels sprouts, and kale. These compounds are derived from glucosinolates when the vegetables are chopped, chewed, or otherwise processed. This natural occurrence is a key factor in their health benefits and nutritional value.
Uses in Industry
Isothiocyanates have found their way into various industrial applications due to their unique properties:
- Pharmaceuticals: Investigated for their potential in cancer prevention and treatment.
- Agriculture: Used as natural pesticides and soil conditioners.
- Chemical Research: Serve as valuable reagents in organic synthesis.
Key Differences
Structural Distinctions
The primary difference between thiocyanate and isothiocyanate lies in their molecular structure. Thiocyanate’s linear arrangement contrasts with isothiocyanate’s arrangement, where the nitrogen atom is directly bonded to carbon. This distinction has a profound impact on their chemical behavior and applications.
Chemical Properties
Due to their structural differences, thiocyanates and isothiocyanates exhibit different chemical properties:
- Reactivity: Isothiocyanates are generally more reactive than thiocyanates, particularly in forming bonds with proteins and other organic molecules.
- Solubility: Their solubility in water and organic solvents varies, affecting their use in different chemical processes.
Biological Effects
The biological effects of these compounds are also notably different:
- Thiocyanates are known to interfere with thyroid function at high concentrations, while also playing a role in blood pressure regulation.
- Isothiocyanates are researched for their anticancer properties, attributed to their ability to induce apoptosis in cancer cells.
Importance in Research
Thiocyanate in Medical Studies
Thiocyanate compounds are under study for their potential therapeutic benefits. They are explored for:
- Antihypertensive effects: Investigating their role in blood pressure management.
- Protective effects: Research into their ability to protect against certain types of oxidative stress.
Isothiocyanate in Cancer Research
Isothiocyanates have attracted significant attention for their anticancer effects. Research focuses on:
- Mechanisms of action: Understanding how these compounds inhibit cancer cell growth.
- Dietary studies: Examining the impact of cruciferous vegetable consumption on cancer risk.
Environmental Impact
Thiocyanate Pollution Sources
Thiocyanate pollution primarily originates from industrial activities such as mining, metallurgy, and the manufacturing of textiles and plastics. These processes can release thiocyanates into water bodies, soil, and the atmosphere, posing risks to environmental health and safety. Notably, thiocyanates are by-products of cyanide usage in gold mining, leading to concerns over water pollution and aquatic life toxicity. The persistence of thiocyanate in the environment depends on conditions such as pH, temperature, and the presence of oxidizing agents, which can affect its breakdown and the severity of its impact.
Isothiocyanate as Biopesticides
Isothiocyanates are emerging as eco-friendly biopesticides due to their natural occurrence in plants and their ability to deter pests. Unlike synthetic pesticides, isothiocyanates degrade into less harmful substances, minimizing their environmental footprint. They work by disrupting the metabolic processes of pests and pathogens, offering a sustainable alternative to chemical pesticides in agriculture and horticulture. The use of isothiocyanate-based biopesticides aligns with the growing demand for organic farming practices and the need to reduce chemical inputs in agriculture.
Safety and Handling
Exposure Risks
Both thiocyanates and isothiocyanates pose exposure risks that require careful handling and safety measures. For thiocyanates, risks include thyroid dysfunction and neurotoxicity at high levels of exposure, commonly through ingestion or occupational contact. Isothiocyanates, while beneficial in dietary amounts, can cause respiratory and skin irritation when handled in concentrated forms. The volatility of certain isothiocyanates also poses inhalation risks, particularly in industrial settings.
Safe Practices
Adopting safe handling practices is essential to minimize exposure risks and ensure the safety of individuals working with these compounds. These practices include:
- Personal Protective Equipment (PPE): Wearing appropriate PPE such as gloves, goggles, and respirators to prevent skin, eye, and respiratory exposure.
- Ventilation: Ensuring adequate ventilation in work areas to dilute airborne concentrations of volatile isothiocyanates.
- Spill Management: Having protocols in place for dealing with spills, including neutralizing agents and containment measures.
Recent Advances
Innovations in Thiocyanate Applications
Recent research has unveiled innovative applications of thiocyanates, pushing the boundaries of their utility in various fields. One notable advancement is the development of thiocyanate-based electrolytes for use in solar cells and batteries. These electrolytes offer improved stability and efficiency, contributing to the advancement of renewable energy technologies. Additionally, thiocyanates are being explored as antimicrobial agents in healthcare, offering potential alternatives to conventional antibiotics and antiseptics.
Breakthroughs with Isothiocyanates
Isothiocyanates have seen significant breakthroughs, particularly in the realm of health and medicine. Research has demonstrated their efficacy in targeting cancer stem cells, offering new avenues for cancer treatment that could overcome resistance to traditional therapies. Furthermore, isothiocyanates are being studied for their potential to mitigate neurodegenerative diseases due to their antioxidant and anti-inflammatory properties. These compounds are also being developed into novel biopesticides that offer targeted pest control without harming beneficial insects, aligning with integrated pest management strategies.
FAQs
What are Thiocyanate and Isothiocyanate?
Thiocyanate and isothiocyanate are chemical compounds that contain sulfur, carbon, and nitrogen. The key difference lies in their structure: thiocyanate has a linear arrangement of its atoms, while isothiocyanate features a different atomic configuration. This structural distinction affects their chemical reactivity and applications in industries such as pharmaceuticals, agriculture, and environmental science.
How are Thiocyanates and Isothiocyanates Used?
Thiocyanates are widely used in the pharmaceutical industry and in the synthesis of various chemicals. They serve as precursors to certain drugs and as stabilizers in the manufacturing process. Isothiocyanates, on the other hand, are known for their anticancer properties and are being studied for their potential in cancer prevention. Additionally, they are used as biopesticides due to their natural occurrence in cruciferous vegetables and their ability to deter pests.
What are the Environmental Impacts of Thiocyanates?
Thiocyanates can be environmental pollutants when released in large quantities, particularly from industrial sources like mining and metal processing. They can affect water quality and aquatic life due to their toxicity at high concentrations. However, controlled use and proper waste management practices can mitigate their environmental impact, highlighting the importance of regulation and monitoring in industrial applications.
Can Isothiocyanates Benefit Human Health?
Isothiocyanates have been extensively researched for their health benefits, especially their anticancer effects. Found naturally in cruciferous vegetables like broccoli and Brussels sprouts, these compounds have been shown to inhibit the growth of cancer cells in laboratory studies. Regular consumption of foods rich in isothiocyanates may lower the risk of certain types of cancer, making them a significant focus of nutritional and medical research.
Conclusion
Exploring the differences between thiocyanate and isothiocyanate reveals a fascinating intersection of chemistry, biology, and environmental science. These compounds, though similar in name, exhibit unique properties that make them invaluable to various fields of study and application. From their critical roles in pharmaceutical formulations and cancer research to their impact on environmental health, understanding these substances offers insight into the complex interplay of chemical structures and their real-world implications.
The importance of distinguishing between thiocyanate and isothiocyanate extends beyond academic interest, touching on the development of safer, more effective drugs, the creation of sustainable agricultural practices, and the advancement of environmental protection efforts. As research continues to uncover the full scope of their capabilities and effects, these compounds will undoubtedly remain at the forefront of scientific inquiry and innovation, highlighting the endless possibilities that emerge from the detailed study of chemical differences.