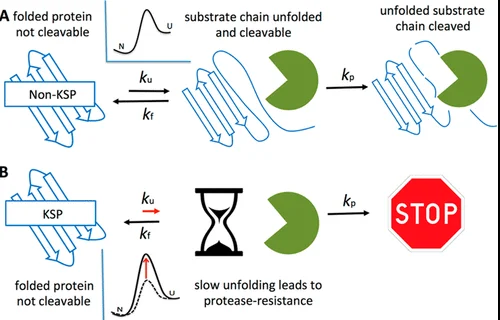
Stability in chemistry refers to the resilience of a compound under specified conditions and the likelihood of its transformation into different compounds. Two fundamental types of stability are often discussed in chemical and physical contexts: thermodynamic and kinetic stability. These concepts are central to understanding how chemical reactions unfold and predict the behavior of substances under various conditions.
Thermodynamic stability is concerned with the energy state of products at equilibrium and whether a reaction is favorable to proceed, while kinetic stability focuses on the rate at which reactions occur. A thermodynamically stable compound is at a lower energy state and less likely to react, whereas a kinetically stable compound does not react quickly, despite possibly being in a higher energy state.
Exploring these two types of stability reveals intricate details about chemical processes, such as why certain compounds are more reactive than others and how this affects everything from material design to pharmaceuticals. The differences between thermodynamic and kinetic stability also help chemists and engineers design safer, more efficient, and sustainable processes and products.
Core Concepts
Stability in Chemistry
Definition of Stability
In the realm of chemistry, stability refers to the ability of a compound to maintain its chemical structure without decomposing or reacting under specific conditions. Stability is not just about being unchangeable; it’s about the resistance to change that a substance exhibits, which can be crucial for everything from pharmaceuticals to industrial products.
Types: Thermodynamic vs Kinetic
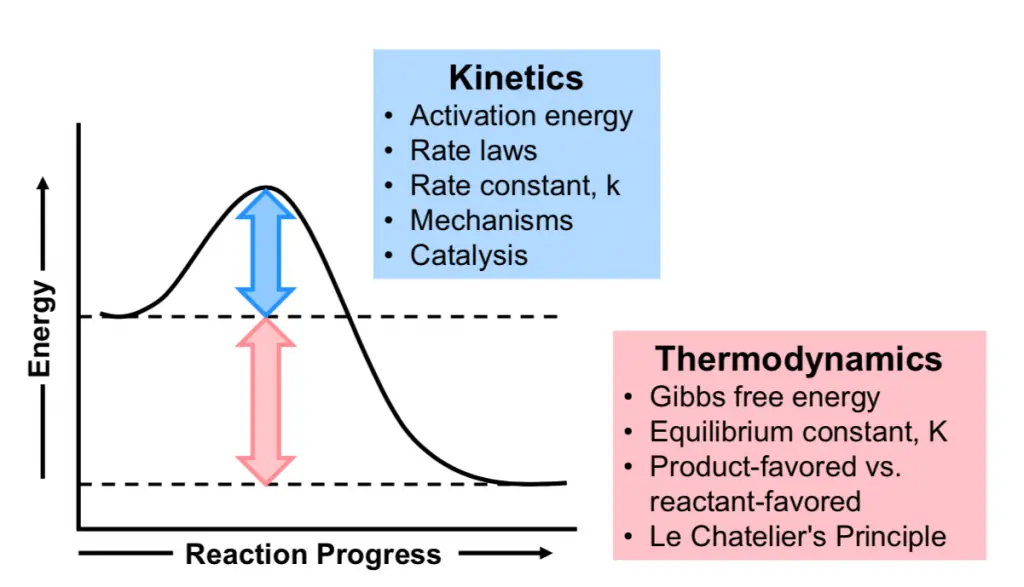
Chemical stability can be classified into two main types: thermodynamic and kinetic. Thermodynamic stability involves the energy state of a compound and its tendency to change or react with time under constant conditions. In contrast, kinetic stability is concerned with the rate at which these reactions occur. Both types of stability provide valuable insights into how substances will behave in different situations.
Thermodynamic Stability
Definition and Fundamentals
Thermodynamic stability refers to the inherent stability of a substance when it reaches equilibrium within its environment. A substance is considered thermodynamically stable if it is in its lowest possible energy state, making it less likely to react or change spontaneously.
How It Is Measured
Measuring thermodynamic stability often involves calculating the Gibbs free energy change (Δ𝐺ΔG) of a reaction. A negative Δ𝐺ΔG indicates that the reaction can occur spontaneously, implying that the products are more stable than the reactants.
Examples in Chemical Reactions
- Combustion of hydrocarbons: The combustion process releases energy, indicating that the products (carbon dioxide and water) are more thermodynamically stable than the hydrocarbon and oxygen reactants.
- Formation of salts: The formation of ionic compounds from metals and non-metals releases energy, suggesting a move towards a more stable energy state.
Kinetic Stability
Definition and Explanation
Kinetic stability refers to the rate at which a chemical reaction progresses towards its final state. A kinetically stable substance may not react quickly, even if it is thermodynamically favorable for it to do so. This type of stability is governed by the activation energy required to initiate the reaction.
Measurement Methods
Kinetic stability is typically assessed by measuring the rate of reaction. Techniques such as spectroscopy or monitoring the change in concentration of reactants or products over time can provide insights into the reaction kinetics.
Examples from Everyday Reactions
- Preservatives in food: These chemicals are kinetically stable, slowing down the rate of decomposition and spoilage.
- Rusting of iron: Although iron is thermodynamically prone to rusting in the presence of oxygen and water, the process can be slow, showing kinetic stability due to the energy barriers involved.
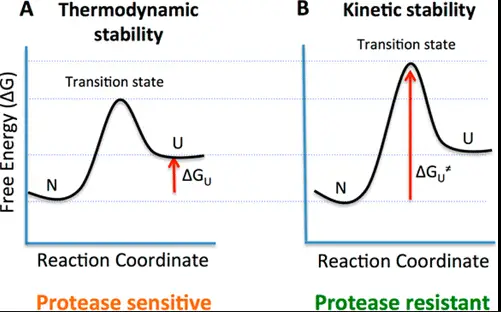
Key Differences
Energy Profiles
Understanding the energy profiles of reactions is crucial for distinguishing between thermodynamic and kinetic stability. Energy profiles illustrate the potential energy of a system as the reaction proceeds.
Energy Wells and Barriers
- Energy well: Represents a state where the system is stable (low energy).
- Energy barrier: The activation energy needed to overcome for the reaction to proceed.
Diagrammatic Representations
Visualizing energy profiles through diagrams helps illustrate the differences in stability:
- Thermodynamic diagrams show the overall energy decrease from reactants to products.
- Kinetic diagrams focus on the height of the energy barrier between reactants and products.
Reaction Rates
Influence on Reaction Speed
The rate of a chemical reaction is intimately linked to kinetic stability. A high activation energy results in a slower reaction rate, reflecting high kinetic stability despite possible thermodynamic favorability.
Examples Comparing Rates
- Explosives like nitroglycerin: Highly thermodynamically unstable due to the vast amount of energy released upon decomposition, yet kinetically stable under normal handling conditions until initiated.
- Diamond turning into graphite: While graphite is more stable thermodynamically, the conversion is extremely slow, indicating high kinetic stability.
Practical Implications
Predicting Product Formation
Understanding both types of stability enables chemists to predict and control the outcome of reactions, crucial for synthetic chemistry and manufacturing processes.
Influence on Industrial Processes
Knowledge of thermodynamic and kinetic stability aids in designing processes that are both efficient and safe, impacting industries ranging from pharmaceuticals to energy production. For example, stabilizing agents can be added to formulations to enhance the shelf life of products or improve the safety of reactive chemicals.
Factors Influencing Stability
Environmental Conditions
Temperature and Pressure Effects
Temperature and pressure are pivotal in determining both thermodynamic and kinetic stability. These environmental variables can significantly alter the state of a substance and its reaction pathways:
- Temperature: Increases in temperature generally increase reaction rates, decreasing kinetic stability. This is due to the increased energy available to overcome activation barriers. Conversely, thermodynamic stability may decrease as higher temperatures can make certain endothermic reactions more favorable.
- Pressure: Changes in pressure, especially in gaseous systems, can alter reaction equilibria according to Le Chatelier’s Principle. Increased pressure typically favors the formation of denser states, influencing the direction and extent of reactions, thus affecting stability.
Catalyst Presence
Catalysts play a critical role in modifying the stability of substances by reducing the activation energy required for a reaction. While they do not affect the thermodynamic properties directly, they can significantly enhance kinetic stability by allowing reactions to proceed at a faster rate or under milder conditions.
Molecular Structure
Atomic Bonds and Arrangements
The type and strength of atomic bonds within a molecule largely dictate its stability:
- Covalent bonds: Stronger covalent bonds contribute to greater thermodynamic stability.
- Ionic bonds: These can offer high stability under dry conditions, but may be less stable in the presence of solvents like water.
Impact of Molecular Complexity
The complexity of a molecule, including the number and arrangement of atoms, can also affect stability:
- Steric factors: Larger, more complex molecules may exhibit higher kinetic stability due to the physical hindrance of reactive sites.
- Electronic effects: Electron distribution within a molecule can stabilize or destabilize it through resonance and inductive effects.
Analyzing Stability
Case Studies
Thermodynamically Stable but Kinetically Unstable Compounds
Examples such as diamond and graphite illustrate this phenomenon. Diamond is kinetically stable at room temperature, maintaining its structure indefinitely, but thermodynamically, graphite is more stable under the same conditions.
Vice Versa: Kinetic vs Thermodynamic
Nitroglycerin is a classic example of a compound that is kinetically stable enough to handle and transport under controlled conditions but is thermodynamically unstable, making it highly explosive under the right triggering conditions.
Predictive Methods
Computational Chemistry Tools
Advanced software and molecular modeling tools allow scientists to predict stability factors by simulating different environmental and molecular conditions without the need for physical experiments. These tools can model reactions and predict stability based on quantum mechanics and thermodynamic calculations.
Laboratory Techniques for Assessment
Several empirical techniques are used to assess stability:
- Calorimetry: Measures the amount of heat released or absorbed during a reaction.
- Spectroscopy: Analyzes how substances interact with electromagnetic radiation to infer stability.
Industry Applications
Pharmaceutical Development
Stability in Drug Formulation
Stability testing is crucial in the pharmaceutical industry to ensure that drugs remain effective and safe throughout their shelf life. Formulation chemists work to enhance both the kinetic and thermodynamic stability of drugs to prevent degradation over time.
Impact on Efficacy and Shelf-Life
The stability of pharmaceutical compounds affects both their efficacy and shelf-life. Unstable compounds may degrade into less effective or potentially harmful products, thus rigorous stability testing is mandated by regulatory agencies.
Energy Production
Stability in Fuel Compounds
In the energy sector, the stability of fuel compounds, such as gasoline, diesel, and biofuels, is vital for safe storage and efficient use. Enhancing the stability of these fuels can lead to more reliable energy sources and fewer emissions.
Relevance to Sustainability
Stable fuel compounds burn more efficiently and produce fewer unwanted byproducts, contributing to more sustainable energy practices. Research into the stability of newer, greener fuel alternatives continues to be a high priority in efforts to reduce the environmental impact of energy production.
Frequently Asked Questions
What is Thermodynamic Stability?
Thermodynamic stability refers to the overall energy of a system at equilibrium. A thermodynamically stable compound is one that is at its lowest energy state within a given set of conditions and is less prone to change into other forms or react with other substances.
What is Kinetic Stability?
Kinetic stability is about the speed at which a chemical reaction proceeds to its final state. A kinetically stable substance reacts slowly, if at all, under normal conditions, even if it is not in the most stable energy state.
How Do Thermodynamic and Kinetic Stability Differ?
While thermodynamic stability focuses on the energy minimum and favorability of reactions, kinetic stability deals with the reaction rate. A substance can be thermodynamically unstable but kinetically stable, meaning it would not spontaneously transform due to a high energy barrier that prevents the reaction.
Why is Understanding Both Stabilities Important?
Understanding both thermodynamic and kinetic stability is crucial for predicting how substances behave under different conditions, which is essential for developing new materials, drugs, and technologies. It allows scientists to manipulate conditions to favor desired reactions and prevent undesirable ones.
Conclusion
The concepts of thermodynamic and kinetic stability are foundational in the field of chemistry, providing crucial insights into the behavior of molecules and the progression of reactions. Recognizing the differences between these types of stability not only enriches our understanding of chemical processes but also enhances our ability to manipulate them for various applications.
These insights are instrumental in numerous fields, from pharmaceuticals to materials science, where the stability of compounds dictates their usability, safety, and effectiveness. As such, a deep appreciation of both thermodynamic and kinetic stability is essential for advancing technology and improving the quality of life through science.