Chemical reactions are fundamental processes that transform substances through the breaking and forming of chemical bonds. Among the various types of reactions, synthesis and substitution stand out due to their distinct mechanisms and widespread applications. These reactions not only play a pivotal role in academic studies but also have practical implications in industries ranging from pharmaceuticals to manufacturing.
A synthesis reaction involves two or more reactants combining to form a single product, typically characterized by simplicity and directness. In contrast, a substitution reaction involves the replacement of an atom or a group within a molecule by another atom or group, often leading to more complex chemical processes. These definitions provide a clear distinction between the two types, each serving unique roles in chemical transformations.
The significance of understanding these reactions extends beyond academic curricula, influencing the development of new materials, medicines, and energy solutions. A detailed exploration of their mechanisms and applications reveals the breadth of their impact, underscoring the importance of these reactions in both nature and industry.

Synthesis Reactions
Definition and Basics
In chemistry, a synthesis reaction is a process where two or more substances combine to form a new, more complex product. This reaction type is fundamental in both organic and inorganic chemistry and is often described using the simple formula: A + B → AB. These reactions are synonymous with direct combination reactions and are integral in the formation of many compounds that are essential to various biological and industrial processes.
Key Characteristics
Synthesis reactions share several distinguishing characteristics:
- Simplicity: They typically involve straightforward mechanisms, with reactants combining to form one product.
- Energy Changes: Most synthesis reactions are exothermic, meaning they release heat. However, this is not universal.
- Reversibility: Under certain conditions, some synthesis reactions can be reversed.
- Requirement for Activation Energy: Like all chemical reactions, a certain amount of energy is required to initiate synthesis reactions.
Common Examples
Examples of synthesis reactions are ubiquitous in daily life and industrial processes:
- Formation of water: 2𝐻2+𝑂2→2𝐻2𝑂2H2+O2→2H2O
- Production of ammonia: 𝑁2+3𝐻2→2𝑁𝐻3N2+3H2→2NH3 (Haber process)
- Manufacturing of sulfuric acid: 𝑆+𝑂2→𝑆𝑂2S+O2→SO2, followed by 2𝑆𝑂2+𝑂2→2𝑆𝑂32SO2+O2→2SO3 in the contact process.
Industrial and Everyday Uses
Synthesis reactions are crucial in various applications:
- Manufacturing: Creating essential chemicals like ammonia and sulfuric acid.
- Construction Materials: Production of cement and other building materials.
- Consumer Products: Synthesis of plastics and synthetic fibers.
Substitution Reactions
Core Concepts
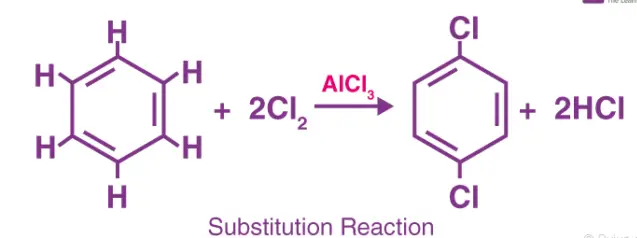
A substitution reaction occurs when an atom or a group of atoms in a molecule is replaced by another atom or group. These reactions are particularly common in organic chemistry and play a crucial role in the modification of molecular structures, which can alter physical and chemical properties.
Distinguishing Features
Substitution reactions are characterized by:
- Types: They can be either nucleophilic or electrophilic, depending on the nature of the substituting species.
- Mechanism Complexity: These reactions can proceed through different mechanisms, such as SN1 or SN2 in organic chemistry.
- Involvement of Intermediates: Some substitution reactions involve the formation of intermediate species or transition states during the reaction process.
Typical Examples
Several everyday chemical reactions are examples of substitution reactions:
- Halogenation of alkanes: 𝐶𝐻4+𝐶𝑙2→𝐶𝐻3𝐶𝑙+𝐻𝐶𝑙CH4+Cl2→CH3Cl+HCl
- Hydrolysis of esters: 𝑅𝐶𝑂𝑂𝑅′+𝐻2𝑂→𝑅𝐶𝑂𝑂𝐻+𝑅′𝑂𝐻RCOOR′+H2O→RCOOH+R′OH
- Exchange reactions in salts: 𝐴𝑔𝑁𝑂3+𝑁𝑎𝐶𝑙→𝐴𝑔𝐶𝑙+𝑁𝑎𝑁𝑂3AgNO3+NaCl→AgCl+NaNO3
Relevance in Organic Chemistry
In organic chemistry, substitution reactions are essential for:
- Synthetic Versatility: They allow chemists to synthesize a wide range of compounds by modifying molecular scaffolds.
- Pharmaceuticals: Many drugs are manufactured using substitution reactions to attach functional groups to core structures.
- Agricultural Chemicals: Pesticides and herbicides often involve substitution reactions during their synthesis.
Comparative Analysis
Reaction Mechanisms
Synthesis and substitution reactions, while both fundamental to chemistry, utilize distinctly different mechanisms. Synthesis reactions typically involve direct combinations where reactants form a more complex product, usually through a single pathway. This process is straightforward, often only requiring the alignment of reactants and sufficient energy to initiate the bond formation.
In contrast, substitution reactions are more complex, involving multiple steps where one functional group in a molecule is replaced by another. These reactions can follow different pathways:
- Nucleophilic substitution is characterized by a nucleophile replacing a leaving group.
- Electrophilic substitution occurs when an electrophile replaces an atom in an aromatic ring.
These mechanisms highlight the intricacies of chemical reactions, where synthesis is more about building new structures and substitution involves altering existing ones.
Energy Requirements
The energy dynamics in chemical reactions are pivotal. Synthesis reactions are typically exothermic, releasing energy as new bonds form. This release is often why such reactions are self-sustaining once started. Substitution reactions, however, can be either exothermic or endothermic, depending on the nature of the substituents and the stability of the transition state.
Reactants and Products
In synthesis reactions, the reactants are usually simple molecules or elements, and the product is more complex. For instance, combining hydrogen and oxygen to form water involves simple diatomic molecules turning into a more complex molecular structure.
For substitution reactions, the reactants are complex molecules that undergo a transformation to replace specific groups, leading to products that are structurally similar but functionally different. An example is the conversion of an alcohol to a halide by replacing the hydroxyl group with a halogen.
Role in Synthetic Chemistry
Both reaction types are critical in synthetic chemistry. Synthesis reactions are essential for creating new compounds from simpler substances, forming the backbone of material synthesis. Substitution reactions are crucial for functionalizing compounds, allowing chemists to tweak molecular structures for desired properties, such as increased efficacy in drugs or enhanced durability in materials.
Visual Guides
Diagrams of Reaction Processes
Visual representations of chemical reactions help clarify complex processes:
- Synthesis reactions can be illustrated where simple reactants combine at a molecular level to form a more complex structure.
- Substitution reactions might show the step-by-step replacement of a functional group, highlighting the intermediate stages and final product.
Side-by-side Comparison
A side-by-side comparison of synthesis and substitution reactions through diagrams can illustrate the differences in their mechanisms, energy changes, and the nature of reactants and products. This visual juxtaposition helps in understanding the distinct roles each reaction type plays in chemistry.
Impact and Applications
In Pharmaceuticals
Synthesis reactions in pharmaceuticals allow for the production of complex drugs from simpler substances, often resulting in potent medicines that can treat various diseases. Substitution reactions are equally important, as they enable the modification of existing drug structures to improve their activity or reduce side effects.
In Manufacturing
In manufacturing, synthesis reactions are used to create polymers, alloys, and other compounds essential for building materials, electronics, and consumer goods. Substitution reactions find applications in creating synthetic dyes, fragrances, and other specialized chemicals that require precise functional attributes.
Environmental Implications
The environmental impact of chemical reactions is significant. Synthesis reactions, while useful, can sometimes produce undesirable byproducts that may harm the environment. Conversely, substitution reactions can be designed to be greener by selecting reactants and conditions that minimize toxic byproducts, thus promoting sustainable chemical practices.
FAQs
What is a synthesis reaction?
A synthesis reaction is a type of chemical process where two or more reactants combine to form a single, more complex product. This type of reaction is fundamental in forming compounds in both laboratory settings and natural processes.
How does a substitution reaction work?
In a substitution reaction, one atom or group in a molecule is replaced by another atom or group. This change typically occurs in organic compounds and is crucial for the modification of molecular structures in synthetic chemistry.
Are synthesis reactions always exothermic?
While many synthesis reactions are exothermic, releasing energy in the process of product formation, this is not a universal characteristic. The energy dynamics can vary based on the reactants involved and the conditions under which the reaction occurs.
Can substitution reactions be reversed?
Substitution reactions can often be reversible, especially in cases where the substituting agent and the original atom or group can compete equally for their position in the molecule, leading to a dynamic equilibrium.
Conclusion
Synthesis and substitution reactions represent crucial pathways through which chemical species evolve and interact. Their study not only enriches our understanding of chemical principles but also enhances our ability to design and synthesize new materials and drugs. These reactions highlight the intricate dance of atoms and molecules that underpins both the natural and the engineered worlds.
The exploration of these reaction types deepens our appreciation for the complexity and beauty of chemical processes. As we continue to uncover the subtleties of synthesis and substitution reactions, we pave the way for innovations in various scientific and industrial fields, promising advancements that could redefine our interaction with the material world.

