Chemical reactions are the heartbeats of the molecular world, driving transformations that underpin everything from the creation of new materials to the processes sustaining life itself. Two fundamental types of these reactions are synthesis and dissociation reactions. They play pivotal roles in the dynamic dance of atoms and molecules, yet operate under very different principles and conditions.
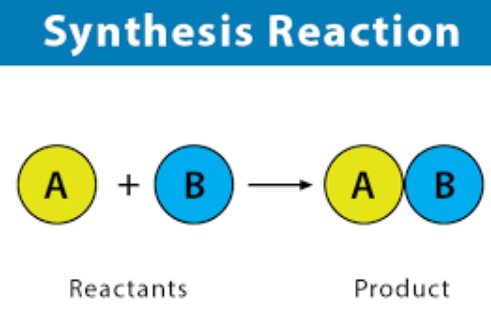
A synthesis reaction involves two or more substances combining to form a more complex product. In contrast, a dissociation reaction is where a compound breaks down into two or more simpler substances. These processes are critical in various scientific and industrial fields, influencing everything from the production of everyday materials to the development of crucial medications.
While synthesis reactions typically require energy to forge new bonds, dissociation reactions often release energy as bonds are broken. The environment, catalysts, and the nature of the reactants themselves can significantly influence these reactions’ direction and speed. Understanding these distinctions not only enriches our knowledge of chemistry but also opens doors to manipulating these reactions for innovative solutions in technology, medicine, and environmental management.
Synthesis Reaction
Definition and Basics
In the realm of chemistry, a synthesis reaction is a fundamental process where two or more reactants come together to form a single, more complex product. This form of chemical reaction is integral to the creation of many substances that are essential to both industrial applications and daily life.
Characteristics
Synthesis reactions share several key characteristics:
- They involve the combination of two or more substances.
- These reactions require energy input, typically in the form of heat, light, or electricity, to initiate bond formation between reactants.
- The outcome is the formation of new chemical bonds, resulting in a product that is more complex than the reactants.
Common Examples
Several examples highlight the role of synthesis reactions in chemistry:
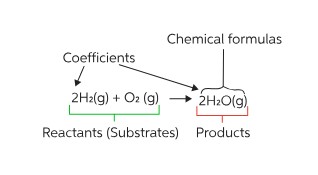
- Water Formation: The combination of hydrogen gas (H₂) with oxygen gas (O₂) to produce water (H₂O) is one of the most fundamental examples of a synthesis reaction.
- Ammonia Synthesis: The Haber process synthesizes ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂), crucial for fertilizers and industrial materials.
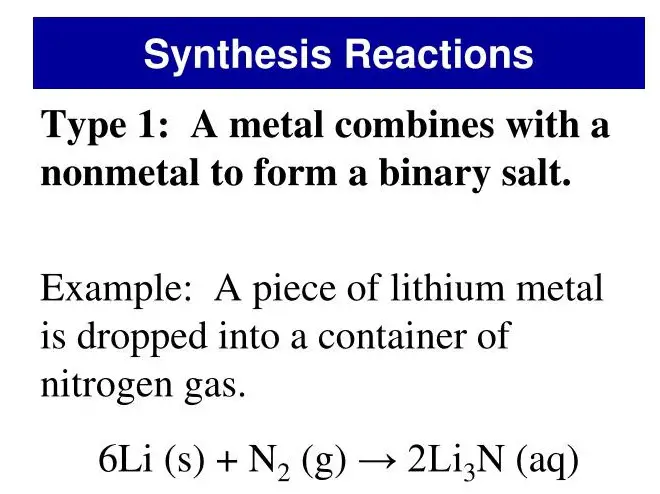
- Synthesis of Salt: The reaction between sodium (Na) and chlorine (Cl₂) to create sodium chloride (NaCl) demonstrates a synthesis reaction producing a daily essential.
Applications in Everyday Life
Synthesis reactions are ubiquitous, with applications spanning:
- Materials Production: From plastics to metals, synthesis reactions are foundational in creating materials for construction, packaging, and manufacturing.
- Pharmaceuticals: The development of drugs relies on synthesis reactions to create complex molecules essential for medicine.
- Agriculture: Synthesizing fertilizers supports crop growth, directly impacting food supply and agriculture.
Dissociation Reaction
Definition and Overview
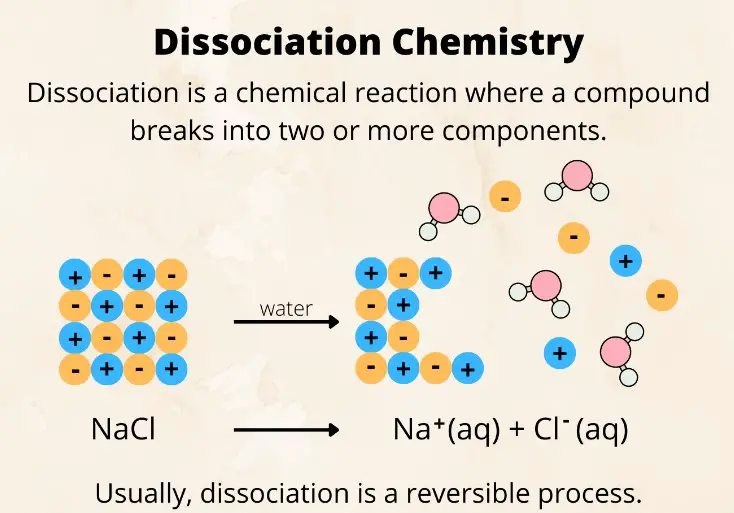
Contrasting with synthesis, a dissociation reaction occurs when a single compound breaks down into two or more simpler substances. These reactions can be spontaneous or may require an energy input, reflecting the diversity of chemical processes in nature and industry.
Key Features
Dissociation reactions are characterized by:
- The breakdown of compounds into simpler elements or compounds.
- Release of energy in many instances, as chemical bonds are broken.
- The role of catalysts in facilitating or accelerating these reactions.
Typical Examples
Examples of dissociation reactions include:
- Water Dissociation: The electrolysis of water into oxygen and hydrogen gases illustrates how energy input can drive the dissociation of molecules.
- Calcium Carbonate Decomposition: Heating calcium carbonate leads to its breakdown into calcium oxide and carbon dioxide, a reaction critical in cement production.
- Acid in Water: The dissociation of hydrochloric acid (HCl) in water to release hydrogen ions (H⁺) and chloride ions (Cl⁻) showcases a common dissociation reaction in chemistry.
Role in Biological Processes
Dissociation reactions play vital roles in biology, including:
- Nutrient Absorption: Breaking down nutrients into absorbable forms.
- Oxygen Transport: The dissociation of oxygen molecules in the bloodstream for cellular use.
- Metabolic Reactions: The breakdown of complex molecules into simpler ones for energy and growth.
Comparative Analysis
Reaction Process
The processes of synthesis and dissociation reactions highlight the dynamic nature of chemical reactions:
- Synthesis: Combines simpler molecules into more complex ones, requiring energy input for bond creation.
- Dissociation: Breaks down complex molecules into simpler ones, often releasing energy as bonds are broken.
Energy Changes
The energy dynamics distinguish these reactions significantly:
- Synthesis Reactions: Typically endothermic, absorbing energy to forge new bonds.
- Dissociation Reactions: Generally exothermic, releasing energy as molecular bonds are severed.
Reactants and Products
The nature of reactants and products in these reactions underscores their differences:
- Synthesis: Starts with simple reactants to end with a complex product.
- Dissociation: Begins with a complex molecule that breaks down into simpler products.
Environmental Conditions
Environmental factors play crucial roles in these reactions:
- Synthesis: May require specific temperatures, pressures, or catalysts to proceed.
- Dissociation: Conditions such as temperature, presence of light, or catalysts can drive or accelerate the reaction.
Factors Influencing Reactions
Chemical reactions are influenced by a variety of factors that can affect their rate, direction, and overall success. Understanding these factors is crucial for scientists and industries that rely on precise chemical outcomes.
Temperature
Temperature plays a pivotal role in chemical reactions. It affects the kinetic energy of the molecules involved, which in turn influences the reaction rate. For most reactions:
- Higher temperatures increase the molecules’ kinetic energy, leading to more frequent and more energetic collisions. This often speeds up the reaction.
- Lower temperatures decrease these interactions, slowing down the reaction.
Concentration
The concentration of reactants is another critical factor:
- A higher concentration of reactants leads to more collisions per unit time, which can accelerate the reaction.
- Conversely, a lower concentration tends to slow down the reaction due to fewer collisions.
Catalysts
Catalysts are substances that increase the reaction rate without being consumed in the process. They work by:
- Providing an alternative pathway for the reaction with a lower activation energy.
- Not altering the reactants’ concentration or the final products.
Practical Applications
Chemical reactions, whether synthesis or dissociation, find applications in various sectors, from industry to environmental science and pharmaceutical development.
Synthesis Reactions in Industry
In industry, synthesis reactions are fundamental for producing a wide range of materials and chemicals:
- Manufacturing Plastics: Synthesis reactions are used to polymerize monomers into polymers, creating various plastics used worldwide.
- Producing Chemicals: Essential chemicals for agriculture, such as fertilizers and pesticides, are often produced through synthesis reactions.
Dissociation Reactions in Environmental Science
Dissociation reactions have significant roles in environmental science:
- Ozone Layer Regeneration: The dissociation of chlorine monoxide in the stratosphere, a reaction that helps regenerate ozone molecules, is crucial for protecting the Earth from UV radiation.
- Water Treatment: Dissociation reactions are used to remove contaminants from water, improving its quality for consumption and use in agriculture.
Interplay in Pharmaceutical Development
The development of pharmaceuticals often involves both synthesis and dissociation reactions:
- Drug Synthesis: Active pharmaceutical ingredients (APIs) are synthesized through complex reactions that often involve multiple steps of synthesis.
- Metabolism Studies: Understanding how drugs dissociate and metabolize in the body is essential for developing safe and effective medications.
Challenges and Solutions
Working with chemical reactions presents several challenges, from handling reactive substances to controlling reaction conditions. Innovations in reaction monitoring and management are crucial for overcoming these challenges.
Handling Reactive Substances
Reactive substances can pose risks of explosions, fires, and toxic exposures. Safe handling practices include:
- Proper Storage: Keeping reactive substances in conditions that minimize the risk of accidental reactions.
- Safety Protocols: Implementing strict safety protocols and training for personnel handling these substances.
Controlling Reaction Conditions
Precise control over reaction conditions such as temperature, pressure, and pH is vital for achieving desired outcomes:
- Automated Control Systems: Using technology to monitor and adjust reaction conditions in real-time, ensuring consistent results.
- Advanced Reactors: Developing reactors capable of maintaining optimal conditions for specific reactions, improving efficiency and safety.
Innovations in Reaction Monitoring
Technological advancements have led to better ways of monitoring reactions:
- Real-Time Analytics: Techniques like spectroscopy and chromatography allow for the real-time monitoring of reaction progress, providing immediate data on reaction rates and product formation.
- Machine Learning: Incorporating machine learning algorithms to predict reaction outcomes, optimize conditions, and even discover new reaction pathways.
Frequently Asked Questions
What is a Synthesis Reaction?
A synthesis reaction, also known as a combination reaction, is a chemical process where two or more reactants combine to form a single, more complex product. This type of reaction is fundamental in creating many compounds used in various industries, from manufacturing polymers to developing pharmaceuticals. Energy is typically absorbed to form new bonds, illustrating the process’s significance in building the structural complexity in chemistry.
How does a Dissociation Reaction work?
In a dissociation reaction, a compound breaks apart into two or more simpler substances, often as a response to heating, radiation, or the presence of another substance. This process is crucial in many biological and chemical systems, facilitating processes such as nutrient absorption in living organisms and the breakdown of compounds in environmental cycles. Energy is usually released as existing bonds are broken.
Why are Synthesis and Dissociation Reactions important?
Synthesis and dissociation reactions are central to chemical processes, playing vital roles in the natural world and human-made technologies. They allow for the transformation of substances into new forms, enabling the creation of a wide range of products from medicines to materials. Understanding these reactions is key to advancements in chemistry, environmental science, and pharmacology, highlighting their importance in innovation and sustainability.
Conclusion
The exploration of synthesis and dissociation reactions unveils the intricate balance and complexity inherent in chemical processes. These reactions not only underscore the fundamental principles of chemistry but also have far-reaching implications across various scientific disciplines and industries. Their study and manipulation pave the way for advancements in technology, medicine, and environmental conservation, reflecting the power of chemistry to transform and enrich our world.
By grasping the nuances of these reactions, we equip ourselves with the knowledge to innovate and solve complex problems. The continuous study and application of synthesis and dissociation reactions hold the promise of future discoveries and developments that can further our understanding of the universe and improve the quality of life across the globe.