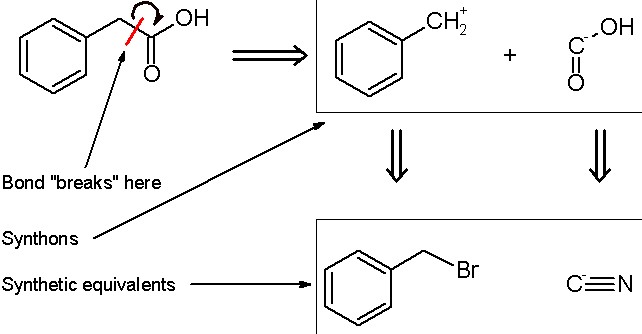
Chemical synthesis and retrosynthesis stand as twin pillars in the vast and intricate world of chemistry, each playing a critical role in the development and understanding of new compounds, particularly in pharmaceuticals. While they share a common goal, the approach and execution differ significantly, offering unique insights and challenges. Synthesis involves the combination of elements and compounds to form more complex molecules, a forward-looking process that builds up to the desired compound. Retrosynthesis, on the other hand, starts with the target molecule and works backwards to discern simpler precursor molecules from which it can be synthesized.
The key difference between synthesis and retrosynthesis lies in their directional approaches to molecule construction. Synthesis is a forward process, constructing complex molecules from simpler ones, whereas retrosynthesis is a methodical reverse-engineering process, breaking down complex molecules into simpler, more manageable components to understand how they can be assembled from available substances.
Both processes are indispensable in the field of chemistry, especially in drug design and materials science. Synthesis allows chemists to create new molecules, exploring the boundaries of what can be made. Retrosynthesis, conversely, offers a strategic blueprint for deconstructing complex molecules, facilitating the design of efficient synthesis pathways for compounds whose constructions were previously unknown or deemed too challenging.
Synthesis Explained
Basics of Synthesis
In the realm of chemistry, synthesis refers to the process of combining simpler substances to form more complex molecules. This foundational technique is the cornerstone of countless innovations in science, allowing researchers to fabricate materials and compounds with specific, desired properties. At its core, synthesis transforms raw elements or compounds through a series of chemical reactions, culminating in the production of a new, complex substance.
Types of Synthesis
There are several types of synthesis, each with its unique methods and applications. Let’s explore the most common ones:
Organic Synthesis
Organic synthesis focuses on constructing organic molecules, crucial for developing pharmaceuticals, agrochemicals, and polymers. This method often involves making carbon-carbon bonds, utilizing catalysts, and controlling stereochemistry to achieve the desired molecular structure.
Inorganic Synthesis
Inorganic synthesis is concerned with the creation of inorganic compounds, excluding organic molecules. It plays a vital role in developing materials such as ceramics, metals, and semiconductors, crucial for electronics and materials science.
Biochemical Synthesis
Biochemical synthesis is pivotal in biochemistry, involving the formation of complex biological molecules. Enzymes often catalyze these reactions, leading to the production of substances like DNA, proteins, and carbohydrates, essential for life.
Role in Industry
Synthesis has a profound impact across various industries, notably in pharmaceuticals, materials science, and technology:
- Pharmaceuticals: Synthesis is crucial for creating active pharmaceutical ingredients (APIs) and intermediates, leading to the development of new drugs.
- Materials Science: It allows for the creation of new materials with tailored properties, such as improved strength, conductivity, or biocompatibility.
- Technology: Synthesis contributes to advancements in electronics, through the production of semiconductors and nanomaterials, enhancing device performance and functionality.
Retrosynthesis Uncovered
Basics of Retrosynthesis
Retrosynthesis is the process of deconstructing a complex molecule into simpler precursors, effectively reversing the synthetic process. It is a critical strategy in organic chemistry, enabling chemists to plan the synthesis of complex molecules by breaking them down into more manageable, synthesizable units.
Strategy and Steps
The approach to retrosynthesis involves several key steps:
- Identify Functional Groups: Recognize the functional groups in the target molecule.
- Disconnection: Conceptually “break” the molecule into simpler pieces.
- Choose Starting Materials: Select readily available or easily made starting materials.
- Synthesis Plan: Develop a step-by-step plan to synthesize the target molecule from the chosen starting materials.
Role in Drug Design
Retrosynthesis is indispensable in drug design, providing a roadmap for synthesizing new medicinal compounds. It aids in discovering the most efficient synthesis routes, potentially reducing production costs and time. This backward approach is crucial for innovating new treatments and expediting their availability to the market.
Key Differences
Conceptual Approach
The fundamental difference between synthesis and retrosynthesis lies in their directional approaches. Synthesis is a forward process, building complex molecules from simpler ones. In contrast, retrosynthesis is a reverse-engineering process, breaking down complex molecules to understand how they can be constructed from simpler components.
Application Areas
While both synthesis and retrosynthesis are applied across chemistry and related fields, their specific applications can differ. Synthesis is directly involved in the creation of new materials and drugs, driving forward innovations. Retrosynthesis, however, is more about planning and optimization, playing a crucial role in understanding and improving the efficiency of synthetic routes.
Complexity and Creativity
Both processes demand a high level of creativity and understanding of chemical complexity. Synthesis requires innovation in combining molecules in new ways, while retrosynthesis demands ingenuity in dissecting complex structures into their elemental building blocks. This interplay of creativity and complexity is what propels advancements in chemistry and related disciplines.
Practical Examples
Case Study: Drug Synthesis
Aspirin Synthesis
Aspirin, also known as acetylsalicylic acid, is a widely used medication for pain relief, fever reduction, and anti-inflammation. Its synthesis is a classic example of chemical synthesis from base chemicals, showcasing the principles of organic chemistry in action. The process involves the reaction between salicylic acid and acetic anhydride, facilitated by an acid catalyst such as sulfuric acid or phosphoric acid.
- Reactants: Salicylic acid + Acetic anhydride
- Catalyst: Sulfuric acid (H2SO4) or Phosphoric acid (H3PO4)
- Product: Aspirin (Acetylsalicylic acid)
This synthesis exemplifies how simple chemical principles can be applied to create compounds of significant medicinal value.
Case Study: Drug Retrosynthesis
Aspirin Retrosynthesis
Retrosynthetic analysis of aspirin begins by identifying its core components: a benzene ring, a carboxylic acid group (COOH), and an ester group (OCOCH3). The goal is to determine simpler precursor molecules that can be used to synthesize aspirin. The analysis might reveal that salicylic acid, possessing both the benzene ring and the carboxylic acid group, is a suitable starting point. The ester group can then be introduced through acetylation with acetic anhydride. This retrosynthetic approach provides a clear, logical pathway to synthesize aspirin, showcasing the power of working backward from the target molecule.
Challenges and Solutions
Challenges in Synthesis
Synthesis faces several obstacles, including:
- Selectivity: Achieving the right reaction pathway among multiple possibilities.
- Yield: Maximizing the amount of desired product.
- Scalability: Transferring the synthesis from lab scale to industrial scale without loss of efficiency or product quality.
Challenges in Retrosynthesis
Retrosynthetic analysis also encounters specific challenges:
- Complexity: Deconstructing molecules with multiple functional groups or stereocenters.
- Pathway Identification: Finding the most efficient synthetic route among many possibilities.
- Starting Material Availability: Ensuring the starting materials identified are readily available or synthesizable.
Overcoming Challenges
Strategies and technologies have evolved to address these challenges:
- Catalysis: Catalysts increase reaction selectivity and yield.
- Computational Chemistry: Software tools predict reaction outcomes and assist in pathway planning.
- Green Chemistry: Principles that emphasize safer, more sustainable chemical processes.
Future Perspectives
Innovations in Synthesis
Recent trends and technologies shaping the future of synthesis include:
- Flow Chemistry: Continuous reactions that enhance safety, scalability, and efficiency.
- Photochemistry: Using light to drive reactions, opening up new reaction pathways.
- Automated Synthesis: Robotics and AI algorithms optimize synthesis procedures and discover new reactions.
Innovations in Retrosynthesis
Advancements in retrosynthetic analysis focus on:
- AI and Machine Learning: Algorithms that can predict retrosynthetic pathways, improving efficiency and accuracy.
- Database Expansion: Comprehensive databases of chemical reactions aid in identifying novel pathways.
- Collaborative Platforms: Online platforms where chemists share insights and strategies for complex synthesis challenges.
Impact on Industry
These innovations are poised to transform the pharmaceutical and materials science industries by:
- Speeding Up Drug Development: Faster, more efficient synthesis routes reduce the time from discovery to market.
- Reducing Costs: Improved processes and automation lower production costs.
- Enhancing Sustainability: Greener synthesis methods minimize environmental impact.
Frequently Asked Questions
What is Chemical Synthesis?
Chemical synthesis involves constructing complex chemical compounds from simpler ones. It’s a fundamental process in chemistry that enables the creation of a vast array of materials and molecules, serving as the backbone of pharmaceuticals, materials science, and industrial chemistry. By manipulating the conditions under which chemical reactions occur, scientists can produce desired compounds with high specificity and yield.
How Does Retrosynthesis Work?
Retrosynthesis works by taking a target molecule and systematically deconstructing it into simpler precursor compounds. This reverse-engineering process helps chemists identify the most efficient pathway to synthesize a complex molecule, considering both the availability of starting materials and the feasibility of reactions. It’s akin to solving a puzzle, where the final image is known, and the challenge is to find the best way to piece it together from the start.
Why is Retrosynthesis Important in Drug Design?
Retrosynthesis is crucial in drug design because it allows scientists to discover efficient methods for synthesizing new and complex pharmaceutical compounds. By understanding how a drug can be deconstructed into simpler components, researchers can identify novel synthesis pathways, reduce production costs, and improve drug availability. This methodical approach is essential for the rapid development and optimization of drugs, making treatments more accessible and affordable.
How do Synthesis and Retrosynthesis Complement Each Other?
Synthesis and retrosynthesis complement each other by offering forward and backward perspectives on molecule construction. While synthesis builds up molecules from simpler substances, retrosynthesis breaks them down to find the optimal synthesis route. This dual approach enhances our understanding of chemical reactions, increases efficiency in creating complex molecules, and accelerates the development of new drugs and materials. Together, they form a comprehensive strategy for tackling the challenges of molecular chemistry.
Conclusion
The interplay between synthesis and retrosynthesis represents a fundamental dynamic in the pursuit of chemical knowledge and innovation. While synthesis propels us forward, creating new molecules and exploring the uncharted territories of chemistry, retrosynthesis offers a reflective lens, guiding us through the complexities of molecular structures to uncover simpler, more efficient paths to their creation. This balance between construction and deconstruction enriches our understanding and facilitates advancements in various fields, especially pharmaceuticals.
As we continue to push the boundaries of what can be synthesized, retrosynthesis will remain an indispensable tool, ensuring that every forward step is grounded in a deep understanding of how complex molecules can be assembled from their elemental roots. Together, synthesis and retrosynthesis not only expand our chemical repertoire but also streamline the path to new discoveries, making the future of chemistry as promising as it is exciting.