Sulfur hexafluoride (SF6) and disulfur tetrafluoride (S2F4) are two chemical compounds with significant roles in various industrial and scientific fields. Each possesses unique properties and applications that make them invaluable in their respective domains. While they share a common element—sulfur—their differences in chemical composition and structure lead to distinct behaviors and uses.
The key difference between sulfur hexafluoride and disulfur tetrafluoride lies in their molecular structures and applications. SF6 is a non-toxic, inert, and potent greenhouse gas used primarily as an electrical insulator and arc suppressant, while S2F4 is known for its role in chemical synthesis, with properties that make it less stable and more reactive than SF6.
Understanding these compounds requires a closer look at their chemical makeup, production methods, and physical properties. Sulfur hexafluoride’s stability and inertness underpin its widespread use in the electrical industry, whereas disulfur tetrafluoride’s reactivity is harnessed in specialized chemical processes. Both, however, pose environmental and safety considerations that necessitate careful handling and regulation.
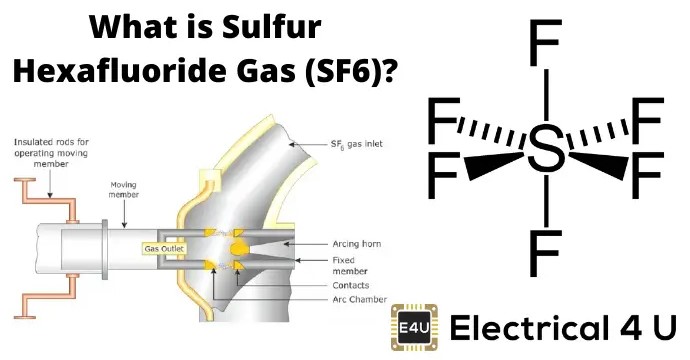
Chemical Structure
SF6 Composition
Sulfur hexafluoride, with its chemical formula SF6, is a compound that combines sulfur with fluorine, featuring one sulfur atom hexagonally surrounded by six fluorine atoms. This configuration grants SF6 its unique characteristics: stability, non-toxicity, and inertness under typical conditions. Its molecular structure is octahedral, showcasing a perfect symmetry that contributes to its physical properties such as its exceptional insulation capability and dense gaseous form.
Physical properties of SF6 that stand out include its heavy gas nature, colorless and odorless presentation, and its remarkable stability which prevents it from reacting with most substances. These attributes make SF6 an ideal candidate for a variety of applications, especially in the electrical industry where it is utilized as an insulating gas.
S2F4 Composition
Disulfur tetrafluoride, or S2F4, presents a chemical structure that is less symmetrical compared to SF6. It consists of two sulfur atoms bonded together, with two fluorine atoms attached to each sulfur. This structure is pivotal for S2F4’s reactivity, making it significantly more reactive than SF6. The molecule’s configuration is crucial in determining its physical and chemical properties.
Physical properties of S2F4 include its gaseous state at room temperature, a pungent odor, and its propensity to react under certain conditions, unlike the inert SF6. These properties are key to understanding S2F4’s applications and handling requirements in industrial settings, highlighting the importance of careful management when dealing with reactive gases.
Production Methods
SF6 Synthesis
The production of sulfur hexafluoride can be achieved through a few industrial processes, the most common being the direct fluorination of sulfur using fluorine gas. The steps involved in this process are as follows:
- Heating sulfur in the presence of fluorine gas.
- The reaction that follows is highly exothermic, forming SF6 as a result.
- Purification of the produced SF6 gas to remove any byproducts or impurities.
This method ensures the production of high-purity SF6, which is essential for its use in sensitive applications such as electrical insulation and arc quenching in circuit breakers.
S2F4 Synthesis
Producing disulfur tetrafluoride involves a more complex chemical process, reflecting its reactivity and the need for precision in its synthesis. The production method typically includes:
- The reaction of sulfur dichloride with sodium fluoride or potassium fluoride.
- Controlled temperature and pressure conditions to facilitate the reaction while preventing unwanted byproducts.
- Purification stages to achieve high-purity S2F4 gas.
These steps underscore the reactive nature of S2F4 and the careful control required during its production, differentiating it from the more straightforward synthesis of SF6.
Physical Properties
SF6 Properties
Sulfur hexafluoride is notable for its heavy molecular weight and density, making it heavier than air. This property is crucial for its application in the electrical industry, where it serves as an effective insulator. Other significant physical properties include:
- A boiling point of -64°C, indicating its gaseous state under normal atmospheric conditions.
- It is colorless and odorless, making it undetectable without specialized equipment.
- Inertness, allowing it to remain stable and non-reactive to most substances.
These properties make SF6 an ideal insulating gas, used to prevent electrical discharges in high-voltage systems and equipment.
S2F4 Properties
In contrast, disulfur tetrafluoride’s physical properties signify its more reactive nature. These properties include:
- A lower density compared to SF6, yet heavier than air.
- A distinctive, pungent odor that can aid in leak detection.
- More reactive than SF6, with a propensity to decompose in the presence of water vapor, releasing toxic substances.
Applications
SF6 Uses
Sulfur hexafluoride (SF6) serves as a cornerstone in the electrical industry, primarily due to its insulating properties, which prevent electrical discharges in high-voltage equipment. Beyond this, SF6’s applications are diverse:
- Electrical Insulation: SF6 is used in gas-insulated switchgear (GIS), gas-insulated lines (GIL), and transformers, providing effective insulation and reducing the equipment size.
- Arc Quenching: In circuit breakers, SF6 gas extinguishes electrical arcs during the operation of switching off circuits, ensuring the equipment’s safety and longevity.
- Medical Imaging: In ultrasound imaging, SF6 gas is used as a contrast agent due to its ability to improve the clarity of ultrasound images.
- Leak Detection: Its inertness makes SF6 an ideal tracer gas for detecting leaks in various systems, including pipelines and storage containers.
These uses highlight SF6’s versatility across different sectors, emphasizing its critical role in modern technology and industry.
S2F4 Uses
Disulfur tetrafluoride (S2F4), while less widely used than SF6, plays a crucial role in certain chemical synthesis applications due to its reactivity. Key uses include:
- Chemical Synthesis: S2F4 is employed as a reagent in the synthesis of organofluorine compounds, pivotal in pharmaceuticals and agrochemicals.
- Specialty Polymers: In the production of fluorinated polymers, S2F4 can be used to introduce sulfur-fluorine groups, enhancing the material’s properties.
S2F4’s applications are indicative of its niche role, where its reactivity is harnessed for specific chemical processes.
Environmental Impact
SF6 and the Environment
The environmental impact of SF6 is a growing concern, primarily due to its greenhouse gas potential. Key points include:
- Greenhouse Gas: SF6 is one of the most potent greenhouse gases, with a global warming potential many times that of CO2.
- Atmospheric Lifetime: It remains in the atmosphere for thousands of years, contributing to long-term climate change.
- Efforts to minimize SF6 emissions focus on recycling and alternative gases for insulation to mitigate its environmental footprint.
S2F4 and the Environment
The environmental considerations for S2F4 revolve around its toxicity and reactivity:
- Toxicity: Upon exposure to water vapor, S2F4 decomposes into toxic compounds, posing risks to both human health and the environment.
- Controlled Use: The use of S2F4 is carefully regulated to prevent accidental releases, with ongoing research into safer alternatives or handling methods.
Understanding the environmental impacts of both gases is crucial for their responsible management and use.
Safety and Handling
SF6 Safety Measures
Handling and storing SF6 safely is essential to prevent leaks and minimize health risks. Key safety measures include:
- Proper Containment: SF6 should be stored in tightly sealed containers and only used in well-ventilated areas.
- Protective Gear: Handlers should wear appropriate safety equipment, including gloves and eye protection, to avoid contact.
- Leak Prevention: Regular inspections and maintenance of equipment containing SF6 to detect and repair leaks promptly.
S2F4 Safety Measures
Due to its reactivity and toxicity, S2F4 requires stringent safety protocols:
- Ventilation: Use in areas with excellent ventilation to prevent accumulation of toxic fumes.
- Protective Equipment: Comprehensive personal protective equipment (PPE), including respirators and protective clothing, is necessary.
- Emergency Procedures: Facilities using S2F4 should have clear procedures for dealing with spills or leaks, including neutralization and ventilation.
Adhering to these safety measures ensures the well-being of individuals and the environment.
Regulatory Aspects
SF6 Regulations
The use and emission of SF6 are regulated under several international and national frameworks due to its environmental impact:
- Kyoto Protocol: SF6 is listed as a greenhouse gas, with signatory countries committing to reduce its emissions.
- Local Regulations: Various countries have implemented measures to monitor, report, and reduce SF6 usage, especially in the electrical industry.
S2F4 Regulations
Regulatory guidelines for S2F4 focus on its safe handling and use, given its potential health risks:
- Workplace Safety Standards: Regulations mandate exposure limits and require facilities to implement safety measures to protect workers.
- Environmental Protection: Guidelines also cover the storage, transport, and disposal of S2F4 to prevent environmental contamination.
Comparison Summary
Comparing SF6 and S2F4, several key differences emerge:
- Applications: SF6’s primary use is in the electrical industry for insulation and arc quenching, while S2F4 is utilized in chemical synthesis and polymer production.
- Environmental Impact: SF6 is a significant greenhouse gas, contributing to global warming, whereas S2F4’s main concern is its toxicity and reactivity.
- Safety and Handling: Both gases require careful handling, but SF6’s inert nature makes it less hazardous than S2F4 in terms of chemical reactivity.
- Regulations: While both are regulated, SF6 faces stricter controls due to its environmental effects, compared to the health and safety-focused regulations for S2F4.
Frequently Asked Questions
What is Sulfur Hexafluoride Used For?
Sulfur hexafluoride is primarily used in the electrical industry as an insulating gas in high-voltage switchgear. Its excellent dielectric properties and non-toxic nature make it a preferred choice for insulating and quenching arcs in circuit breakers and other electrical equipment. Additionally, SF6 finds applications in medical imaging and as a tracer gas for leak detection.
How is Disulfur Tetrafluoride Produced?
Disulfur tetrafluoride is produced through chemical reactions involving sulfur compounds and fluorine sources. The specific method often involves the fluorination of sulfur dioxide under controlled conditions. This process yields S2F4, which is then purified for use in various chemical synthesis applications, demonstrating its role in producing other fluorinated compounds.
What are the Environmental Impacts of SF6 and S2F4?
Both SF6 and S2F4 have environmental impacts, with SF6 being a potent greenhouse gas that contributes to global warming. Its long atmospheric lifetime and high global warming potential make it a concern for climate change. On the other hand, S2F4’s environmental impact is less about global warming and more about its potential toxicity and the need for careful handling to prevent releases that could harm the environment or human health.
How are SF6 and S2F4 Regulated?
Regulations for SF6 focus on limiting emissions due to its greenhouse gas potential. Various international and national guidelines dictate its use, recovery, and disposal in the electrical industry. For S2F4, regulations are more concerned with occupational safety and environmental protection, aiming to manage its storage, handling, and disposal to mitigate its reactive hazards.
Conclusion
Sulfur hexafluoride and disulfur tetrafluoride, despite their shared elemental base, diverge significantly in their properties, applications, and environmental considerations. The distinction between these two compounds underscores the complexity and specificity of chemical applications in modern industry and science. Their respective roles, from electrical insulation to chemical synthesis, highlight the nuanced understanding required to utilize such substances effectively and responsibly.
The ongoing study and regulation of SF6 and S2F4 reflect a broader commitment to balancing technological advancement with environmental stewardship and safety. As industries continue to evolve, the informed and careful application of such chemicals remains paramount, ensuring that their benefits are harnessed while minimizing their impact on our planet and its inhabitants.
