In the realm of chemical processes, the creation and utilization of solutions stand as fundamental components. These solutions, specifically stock and standard solutions, serve as the backbone of experimental accuracy and repeatability in both academic and industrial laboratories. The precise preparation and application of these solutions are critical for the success of chemical analyses and reactions.
Stock solutions are highly concentrated liquids prepared for the purpose of being diluted to lower concentrations for actual use. In contrast, standard solutions are those of a known concentration used to calibrate instruments or for precise chemical analysis. This distinction is crucial for ensuring experimental accuracy and reliability across various scientific disciplines.
The knowledge of stock and standard solutions, including their preparation, storage, and application, is essential for professionals and students in the field of chemistry. Their roles, although distinct, complement each other in contributing to the precision and accuracy of experimental outcomes. Understanding the differences between these two types of solutions can significantly enhance the efficiency and effectiveness of laboratory work.

Stock Solutions
Basics
Definition
A stock solution is a concentrated solution that serves as a starting point for making diluted solutions. It’s like having a potent syrup that you dilute with water to get your desired taste but in the realm of chemistry.
Purpose in Laboratories
Stock solutions play a critical role in laboratories. They offer a convenient way to create solutions of a consistent concentration. Imagine needing the same solution every day; instead of mixing it from scratch each time, you use a portion of the stock solution and dilute it. This saves time and reduces the margin for error, ensuring reproducibility in experiments.
Preparation
Common Solvents Used
When preparing stock solutions, the choice of solvent is crucial. Water is the most common solvent due to its universal solvent properties. However, for non-polar substances, organic solvents like ethanol, methanol, or dimethyl sulfoxide (DMSO) are used.
Concentration Levels
The concentration of a stock solution is usually high. It’s determined based on the final use of the diluted solution. The idea is to have a concentration that is easily dilutable to meet various experimental needs.
Storage Conditions
Proper storage is key to maintaining the integrity of stock solutions. They are often stored in cool, dark places to prevent degradation. Some are even kept in refrigerators or freezers if they are sensitive to room temperature.
Applications
Role in Experimental Setups
Stock solutions streamline the experimental setup process. By having a pre-made concentrated solution, researchers can quickly prepare diluted solutions as needed, allowing more time for the actual experimentation.
Examples in Various Fields
- In biology, stock solutions of buffers maintain the pH for experiments.
- In chemistry, reagent solutions are kept as stocks for titrations.
- Pharmaceuticals use them to test drug concentrations.
Standard Solutions
Fundamentals
Definition
A standard solution is a solution with a precisely known concentration. It’s used as a reference to determine the concentration of unknown solutions, playing a pivotal role in quantitative analysis.
Role in Quantitative Analysis
The accuracy of quantitative analysis depends significantly on the precision of the standard solutions used. They are the benchmark for calibrating instruments and validating methods, ensuring the reliability of experimental results.
Creating Standard Solutions
Process of Preparation
- Selection of solute: Choose a compound of known purity.
- Weighing: Accurately measure the mass of the solute.
- Dissolution: Dissolve the solute in a solvent, typically water, to a known volume.
- Standardization: Confirm the concentration by comparing it with a solution of known concentration.
Accuracy and Precision in Making
The preparation of standard solutions demands high accuracy and precision. Even minor errors can lead to significant discrepancies in experimental results, highlighting the need for meticulous technique and calibration.
Uses
Calibration of Equipment
Standard solutions are essential for the calibration of analytical instruments like spectrophotometers and titrators, ensuring that these devices produce accurate readings.
Standardization of Other Solutions
They are used to determine the exact concentration of other solutions, which is crucial for experiments where the concentration of reactants affects the outcome.
Key Differences
Concentration
The main difference in concentration between stock and standard solutions is that stock solutions are highly concentrated for later dilution, while standard solutions are prepared at the exact concentration needed for their specific purpose.
Purpose
Stock solutions are designed for convenience and efficiency in preparing other solutions, whereas standard solutions serve as a benchmark for measuring and validating the concentrations of unknown solutions.
Preparation
The preparation of stock solutions focuses on scalability and storage, with less emphasis on precise concentration. On the other hand, standard solutions require meticulous measurement and standardization to ensure their accuracy.
Usage
While both types of solutions are indispensable in labs, they cater to different needs. Stock solutions simplify the process of creating various solution concentrations, and standard solutions are crucial for analytical accuracy in quantitative analysis.
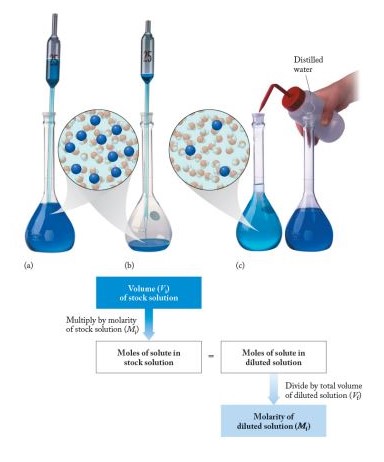
Practical Insights
Choosing the Right Solution
Factors to Consider
When selecting between a stock solution and a standard solution, consider the following:
- Purpose of the Experiment: Determine if accuracy or convenience is the priority.
- Shelf Life: Assess how long the solution will be viable.
- Volume Needed: Larger volumes may benefit from the use of stock solutions.
- Complexity of Preparation: Evaluate the resources and expertise available.
Choosing the right solution streamlines the experimental process, ensuring both efficiency and accuracy.
Adjusting Concentrations
Techniques for Dilution and Concentration
Adjusting the concentration of solutions is a fundamental skill in the lab. Here are simple steps to ensure precision:
- Dilution:
- Calculate the desired concentration and volume.
- Measure a portion of the stock solution.
- Add solvent up to the desired volume, mixing thoroughly.
- Concentration:
- Evaporate the solvent under controlled conditions.
- Monitor the volume until the desired concentration is reached.
These methods allow scientists to tailor solution concentrations for their specific needs.
Safety Measures
Handling and Storage Best Practices
Proper handling and storage of chemicals are vital for safety in the lab. Follow these guidelines:
- Wear Protective Gear: Always use gloves, goggles, and lab coats.
- Label Solutions: Clearly mark all containers with contents and concentration.
- Storage: Store volatile or sensitive chemicals in appropriate conditions, such as in a fume hood or refrigerator.
- Disposal: Follow all regulations for the disposal of chemical waste to protect both people and the environment.
Implementing these safety measures minimizes risks and maintains a healthy working environment.
Case Studies
Industrial Applications
Use in Pharmaceuticals, Manufacturing
In the pharmaceutical industry, the precision of standard solutions is crucial for the formulation and quality control of medications. For example:
- Drug Formulation: Accurate concentrations ensure the therapeutic efficacy and safety of the final product.
- Quality Control: Standard solutions are used to calibrate equipment that measures the potency and purity of drugs.
In manufacturing, stock solutions facilitate the mass production of products, such as in:
- Dyeing Processes: Stock solutions of dyes are diluted to achieve the desired color intensity on fabrics.
- Chemical Synthesis: Reactant solutions are prepared from stocks to ensure consistent quality in products.
These applications demonstrate the critical role of solutions in industrial settings, impacting product quality and manufacturing efficiency.
Academic Research
Role in Scientific Studies
In academic research, both types of solutions are indispensable tools. They enable researchers to conduct experiments with precision and reproducibility, which are the cornerstones of scientific discovery. For instance:
- Analytical Chemistry: Standard solutions are used in titrations to determine unknown concentrations, a fundamental technique in chemical analysis.
- Molecular Biology: Stock solutions of DNA, proteins, and reagents streamline the experimental workflow in genetic studies.
Through these examples, it’s evident that stock and standard solutions are pivotal in advancing scientific knowledge, providing the means to explore complex chemical reactions and biological processes with high fidelity.
Frequently Asked Questions
What is a stock solution?
A stock solution is a concentrated solution that serves as a convenient reservoir for creating solutions of lower concentrations. It is prepared by dissolving a known mass of solute in a solvent to achieve a specific concentration, which can then be diluted as needed for various experiments or analyses.
How is a standard solution prepared?
A standard solution is prepared by accurately weighing a pure substance and dissolving it in a known volume of solvent to achieve a solution of precise molarity. This process often involves volumetric flasks to ensure that the volume of the solution is measured accurately, providing the basis for highly accurate and reliable analytical work.
Why are standard solutions important in analytical chemistry?
Standard solutions are vital in analytical chemistry as they provide a benchmark for measuring the concentration of unknown solutions. They are used to calibrate instruments like spectrophotometers and to conduct titrations, ensuring that experimental results are accurate and reproducible. Without standard solutions, quantifying the concentration of analytes in samples would be fraught with uncertainty.
Can stock solutions be used as standard solutions?
While stock solutions are primarily designed for dilution into more usable concentrations, they can be converted into standard solutions if their concentration is accurately known and verified. This typically involves rigorous measurement and sometimes additional calibration against a primary standard to ensure precision.
Conclusion
Understanding the differences between stock and standard solutions is more than an academic exercise; it’s a practical necessity for anyone involved in chemical analysis or laboratory work. These solutions are the linchpins of accuracy, efficiency, and reliability in experiments, serving distinct but equally vital roles in the scientific process.
Recognizing the importance of these solutions extends beyond their definitions to appreciating their impact on the integrity of experimental outcomes. As such, the knowledge and skills related to preparing and utilizing these solutions are invaluable assets in the toolkit of any chemist or researcher, ensuring the highest standards of scientific inquiry are met.
