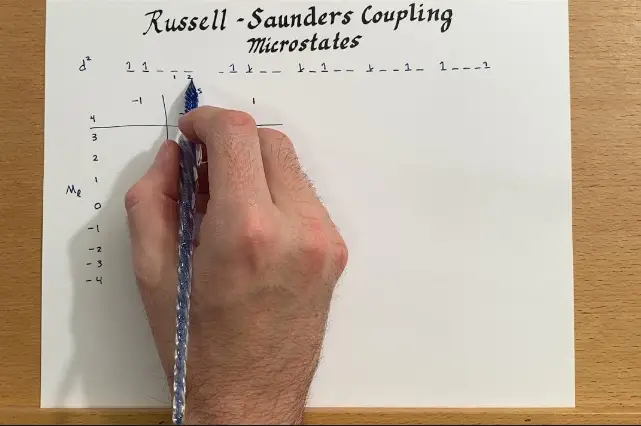
The quantum world of atoms and molecules is complex, and understanding the interaction between their energy levels can be a difficult task. In this blog post, we will discuss two important phenomena in quantum mechanics: spin-orbit coupling and the Russell-Saunders effect.
We will explore the differences between these two phenomena, and what they can tell us about the behavior of atomic and molecular systems.
Explanation of the russell saunders effect
The Russell-Saunders effect is an important concept in atomic physics that explains the connection between the total angular momentum of an atom and the energy of the atom’s energy levels. This effect is distinct from spin-orbit coupling, which describes the interaction between an electron’s spin and its orbital motion within an atom.
This means that the angular momentum of an atom can only correspond to certain energy levels, and any transitions between these energy levels must be accompanied by a change in angular momentum. By contrast, spin-orbit coupling explains how the angular momentum of an atom can change without changing its energy levels.
Comparison between spin orbit coupling and russell saunders effect
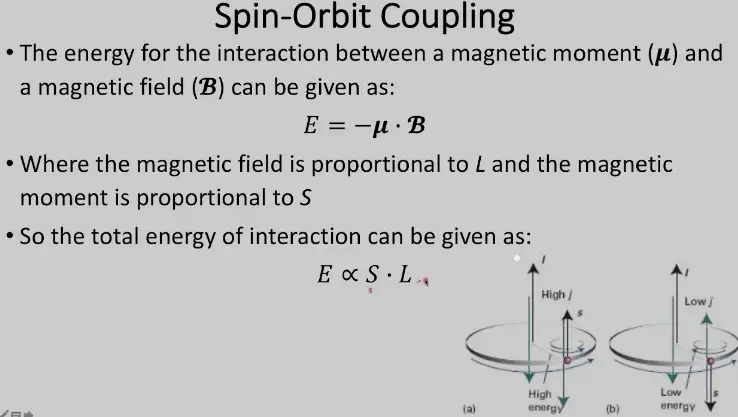
The difference between spin orbit coupling and the Russell-Saunders effect is an important concept to understand in quantum mechanics. Spin orbit coupling describes the interaction between the intrinsic angular momentum of a particle, known as its spin, and the external electrical field that exists in an atom. This interaction results in a splitting of the energy levels of the atom, causing the different spin states of the particle to have different energies.
On the other hand, the Russell-Saunders effect describes the splitting of the energy levels of an atom due to coupling between the orbital angular momentum and the spin angular momentum of the electrons. This coupling is caused by the electrostatic forces between the nuclei and the electrons.
As a result, the energy levels of the atom are split into two sets of energy levels, known as the spin-orbit doublets. This splitting is used to explain the splitting of spectral lines in an atom.
Benefits and drawbacks of spin orbit coupling
The differences between spin-orbit coupling and the Russell-Saunders effect are often overlooked, but they can have a significant impact on our understanding of the physical world. Spin-orbit coupling occurs when the spin and orbital angular momentum of an electron are coupled due to the interaction between the electron and the nucleus of an atom. This phenomenon is particularly evident in heavy atoms, and it results in a splitting of the energy levels of atomic orbitals.
On the other hand, the Russell-Saunders effect is an effect caused by the interaction between the spins of two electrons, which can also result in a splitting of the energy levels of atomic orbitals. The main difference between the two is that spin-orbit coupling is determined by the nucleus of an atom, while the Russell-Saunders effect is determined by the spin of electrons.
As a result, the effects of spin-orbit coupling and the Russell-Saunders effect can have both positive and negative implications in a variety of scientific and technological applications.
Benefits and drawbacks of the russell saunders effect
The Russell-Saunders Effect is a phenomenon that occurs when two different atomic orbitals interact and cause a splitting of the energy levels. This effect is particularly important in the study of atomic spectroscopy, as it is the basis of the selection rules that govern which spectral transitions are allowed.
Although the Russell-Saunders Effect is a useful tool in the study of atoms, it is not the only effect that exists. Another important effect is spin-orbit coupling, which occurs when the angular momentum of an electron is coupled to its orbital angular momentum. The difference between the two effects is that the Russell-Saunders Effect does not consider spin-orbit coupling, whereas spin-orbit coupling does consider the Russell-Saunders Effect.
Both effects can lead to the splitting of energy levels, but the Russell-Saunders Effect is more limited in scope as it does not consider spin-orbit coupling.
Real-world examples of spin orbit coupling and russell saunders effect
When it comes to understanding the differences between spin orbit coupling and the Russell Saunders effect, it is important to understand the real-world examples of each. Spin orbit coupling occurs when the angular momentum of an electron interacts with its orbital motion, resulting in the splitting of atomic energy levels. This phenomenon is also known as spin-orbit splitting.
On the other hand, the Russell Saunders effect occurs when the angular momentum of two electrons in an atom interact with each other, resulting in the splitting of energy levels into two components. In both cases, the result is the same – energy levels split into different components.
However, the underlying mechanisms of each phenomenon are different. Spin orbit coupling is caused by the interaction between the electron’s spin and its orbital motion, while the Russell Saunders effect is caused by the interaction between two electrons’ spins.
Final Touch
In conclusion, spin-orbit coupling and the Russell-Saunders effect are both important concepts in quantum mechanics. The main difference between them is that spin-orbit coupling describes the interaction between the orbital angular momentum of an electron and its intrinsic spin angular momentum, while the Russell-Saunders effect describes the interaction between the total angular momentum of different electrons in an atom. Both of these effects are important in understanding the structure and behavior of atoms and molecules.
Both of these effects are important in understanding the structure and behavior of atoms and molecules.