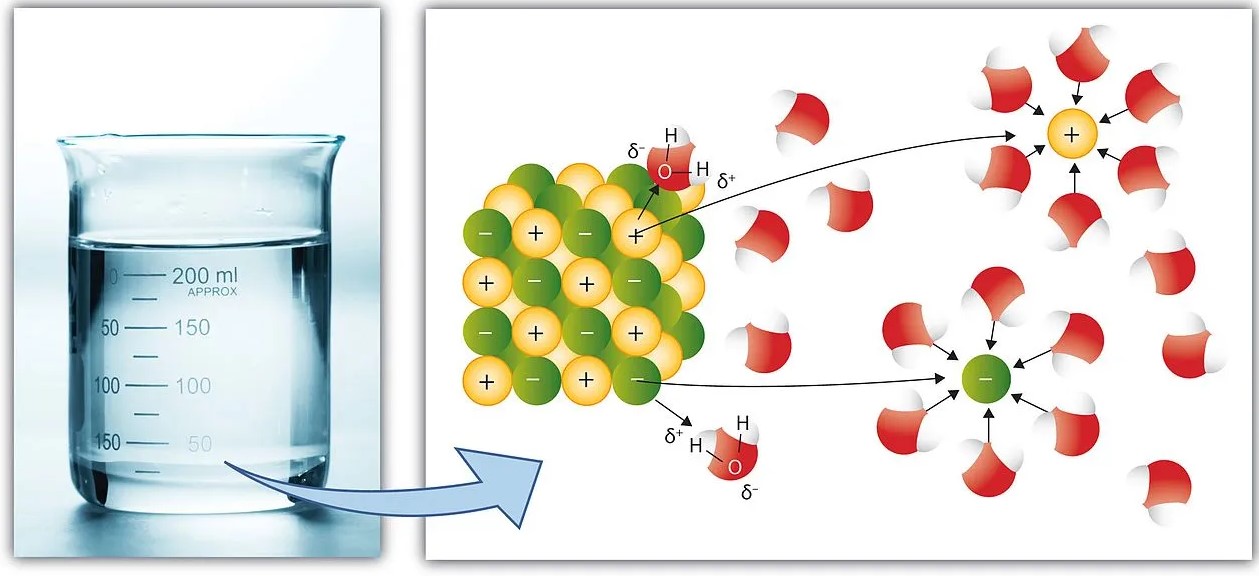
Chemical solutions play a pivotal role in both nature and industry, serving as the medium for countless reactions and processes that sustain life, drive innovation, and form the basis of many technologies. At the heart of these solutions lie two critical concepts: species and phases. Their interplay determines not only the behavior of solutions but also their applications across various fields.
The difference between species and phase in a solution centers on composition and state. Species refer to the individual chemical entities, such as ions, atoms, or molecules, that exist within a solution. Phases, on the other hand, represent the physically distinct sections within a solution that are uniform in chemical composition and physical state. Recognizing this distinction is crucial for understanding how solutions behave and interact under different conditions.
Expanding on these definitions, species in a solution can participate in reactions, affecting the rate and direction of these processes. Phases, characterized by their homogeneity, dictate the physical state of the solution and influence factors such as solubility, conductivity, and density. The interaction between species and phases can dramatically alter the properties of a solution, making this topic a cornerstone of chemical science and its applications.

Species in Solution
Definition and Examples
When discussing chemical solutions, we often refer to the term species. Simply put, a species is any chemical entity that plays a part in the reaction or composition of a solution. This can include ions, atoms, molecules, and even radicals. For example, in a saltwater solution, the sodium (Na⁺) and chloride (Cl⁻) ions are the species within the water (H₂O) solvent.
Types of Species
There are several types of species that can exist in a solution, broadly categorized into:
- Ions: Charged particles that form when an atom or molecule gains or loses electrons. Examples include sodium ions (Na⁺) and chloride ions (Cl⁻).
- Molecules: Groups of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction. Water (H₂O) and glucose (C₆H₁₂O₆) are typical examples.
- Atoms: The basic units of matter and the defining structure of elements. For instance, helium gas in a balloon exists as individual helium atoms.
- Radicals: Highly reactive species with unpaired electrons. Though less common in everyday solutions, they play critical roles in various chemical reactions.
Role in Reactions
In chemical solutions, species are the active participants in reactions. Their interaction determines the rate and direction of the reaction, influenced by:
- Concentration: The more concentrated a species, the higher the chance of collisions and reactions.
- Charge and Polarity: Ions and polar molecules interact strongly with solvents like water, affecting how they react with other species.
- Reactivity: Some species are more reactive than others, determined by their electronic structure.
The presence and concentration of different species in a solution directly impact the outcome of chemical reactions, such as precipitation, acid-base neutralization, and redox reactions.
Phases in Solution
Definition and Clarity
A phase in a solution refers to a physically and chemically homogeneous part of the mixture. Unlike species, which are the individual entities, phases are about the state of matter and uniformity in a solution. Common phases include solid, liquid, and gas.
Examples of Different Phases
In the context of solutions:
- Solid: A phase where the molecules or atoms are closely packed together. Salt in saltwater solution, before it dissolves, is in the solid phase.
- Liquid: The phase where the solution exists in a fluid state, allowing species to move freely. The water in a saltwater solution is the liquid phase.
- Gas: A phase characterized by particles that are far apart and move freely. Carbon dioxide in carbonated water is an example of a gas phase.
Phase Behavior
The behavior of phases in a solution is influenced by several factors, including:
- Temperature: Higher temperatures can change the phase by increasing the kinetic energy of the particles, potentially dissolving more species or transitioning between states (e.g., solid to liquid).
- Pressure: Changes in pressure can also alter the phase, especially for gases, as described by Boyle’s law.
- Composition: The ratio of different species can affect the phase, such as the saturation point of solutes in a solvent.
The presence and stability of phases in a solution impact its properties like boiling point, melting point, solubility, and viscosity.
Key Differences
Nature and Composition
At their core, species and phases represent fundamentally different aspects of a solution. Species are about the individual chemical entities and their roles in reactions, while phases refer to the uniform states of matter within the solution. The distinction is crucial for understanding both the microscopic interactions and macroscopic properties of solutions.
Impact on Solutions
Comparing the effects of species and phases in chemical solutions reveals their unique contributions:
- Species influence the chemical reactivity, determining the types of reactions that can occur, the rate at which they happen, and their outcomes.
- Phases affect the physical properties of the solution, such as its state of matter, solubility, and phase behavior under different conditions.

Measuring and Identifying
Techniques for Species
Understanding what makes up a solution at the molecular or ionic level is crucial for fields ranging from chemistry to environmental science. Techniques have been developed to identify and quantify the species in a solution, each with its unique advantages and applications.
Spectroscopy
Spectroscopy is a powerful tool that analyzes how light interacts with matter. Different species absorb, emit, or scatter light in unique ways, allowing scientists to determine their presence and concentration. The key spectroscopy methods include:
- UV-Visible Spectroscopy: Ideal for identifying species that absorb ultraviolet or visible light, such as metal complexes.
- Infrared Spectroscopy (IR): Useful for identifying molecules based on their bond vibrations, providing insights into molecular structure.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: Determines the structure of organic compounds by observing the behavior of nuclei in a magnetic field.
Chromatography
Chromatography separates components of a mixture based on their movement through a stationary phase. This technique is essential for analyzing complex solutions, identifying components, and quantifying their amounts. Common types include:
- High-Performance Liquid Chromatography (HPLC): Separates and quantifies components in liquid solutions, especially useful for pharmaceutical compounds.
- Gas Chromatography (GC): Best for volatile and gaseous species, often used in environmental analysis and food science.
Electrochemistry
Electrochemistry techniques involve measuring the flow of electrons to or from species in a solution under an applied voltage. Techniques like electrophoresis and potentiometry are widely used to identify ions and molecules based on their charge and electrical activity.
Techniques for Phases
Identifying the phases present in a solution and understanding their distribution and stability are key to many scientific and industrial processes.
Visual Observation
The simplest method, yet often the first step in phase identification, involves observing a solution’s physical state and changes, such as color or phase separation, which can provide immediate insights into the solution’s composition.
Microscopy
Microscopy allows for the direct observation of phases at the microscopic level. Techniques like scanning electron microscopy (SEM) or transmission electron microscopy (TEM) can reveal the arrangement and size of particles in solid phases or emulsions.
Phase Diagrams
Phase diagrams are graphical representations that show the stability of different phases under varying conditions of temperature, pressure, and composition. They are invaluable for predicting phase behavior in mixtures and designing processes for material synthesis and purification.
Applications and Importance
In Laboratory and Industry
The ability to identify species and phases is not just academic; it has real-world applications that impact research, development, and manufacturing across various industries.
Pharmaceuticals
In the pharmaceutical industry, identifying the active pharmaceutical ingredient (API) and its phases is crucial for drug formulation and stability. Techniques like spectroscopy and chromatography are employed to ensure the purity and efficacy of medications.
Materials Science
Understanding the phases present in materials is essential for designing new materials with specific properties. For example, microscopy and phase diagrams are used to develop alloys and polymers with desired strength, flexibility, or conductivity.
Environmental Studies
Identifying pollutants and their concentrations in environmental samples is vital for monitoring and remediation efforts. Electrochemistry and spectroscopy are key tools in detecting and quantifying species like heavy metals or organic compounds in water and soil.
In Everyday Life
The principles of species and phase identification extend into many products and technologies used daily.
Food
The food industry relies on these techniques to ensure product safety and quality. Chromatography and spectroscopy can detect contaminants or verify the authenticity of ingredients, while understanding phase behavior is crucial in the processing and preservation of food.
Medicine
In medicine, identifying the correct form of a drug (its species and phase) can significantly affect its absorption and effectiveness. Techniques such as HPLC and NMR spectroscopy are vital for the analysis and quality control of pharmaceuticals.
Technology
From the batteries that power our devices to the fuels that run our vehicles, understanding the chemical species and phases involved in these technologies allows for improved performance and efficiency. Electrochemistry plays a central role in developing new energy storage solutions, while phase identification is crucial for material engineering.
Frequently Asked Questions
What are chemical species?
Chemical species are individual chemical constituents that make up a solution. They can be atoms, molecules, ions, or radicals, each playing a distinct role in the chemical reactions and interactions that occur within the solution. Understanding the behavior of these species is essential for predicting reaction outcomes, designing chemical processes, and developing new materials.
How do phases affect a solution?
Phases in a solution determine its physical state—whether it’s solid, liquid, or gas—and influence its overall behavior and properties. The phase can affect a solution’s solubility, reactivity, and physical characteristics like density and viscosity. Identifying the phase helps in understanding the conditions under which substances dissolve or precipitate, crucial for chemical engineering and materials science.
Why is distinguishing between species and phases important?
Distinguishing between species and phases in a solution is vital for several reasons. It allows chemists to predict how substances will interact in a mixture, understand the conditions necessary for a reaction to occur, and tailor the properties of a solution for specific applications. This distinction is foundational in fields ranging from pharmaceutical development to environmental chemistry.
Conclusion
Recognizing the distinction between species and phases in a solution is more than an academic exercise; it is a fundamental concept that impacts a wide array of scientific disciplines and practical applications. By understanding the unique roles and behaviors of species and phases, scientists and engineers can manipulate solutions to achieve desired outcomes, from creating new materials to developing life-saving drugs.
This article has shed light on the intricate dance between species and phases within solutions, emphasizing their importance in chemical reactions and industry applications. As we continue to explore and manipulate the microscopic world of chemical solutions, the knowledge of species and phases will remain a crucial tool in our scientific arsenal, driving innovation and discovery across countless fields.