Chemical reactions are the backbone of scientific exploration and industrial innovation, playing pivotal roles in the development of new materials, medicines, and technologies. Among the myriad of reactions, solvolysis and aminolysis stand out due to their specific mechanisms and widespread applications. These processes are fundamental in organic chemistry, contributing significantly to our understanding and manipulation of chemical interactions.
Solvolysis is a reaction in which a solute dissolves in a solvent, leading to the cleavage of a covalent bond in the solute. Conversely, aminolysis involves the substitution of an amine group into a molecule, replacing a leaving group. Both reactions are crucial for the synthesis of various organic compounds, but they operate through distinct mechanisms and are influenced by different factors, including the nature of the solvent and the structure of the reactants.
Focusing on the chemical intricacies, solvolysis typically involves water or alcohol as solvents, facilitating reactions such as ester hydrolysis. Aminolysis, on the other hand, relies on amines to introduce nitrogen-containing groups into molecules, crucial for creating polymers, pharmaceuticals, and agrochemicals. Understanding these reactions provides insights into their roles in chemical synthesis, their environmental implications, and their industrial applications.
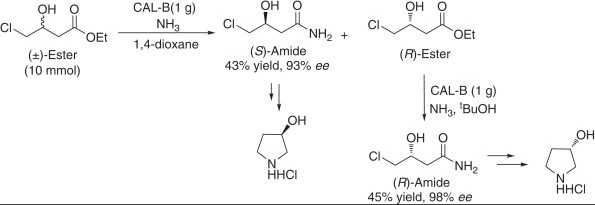
Definitions and Basics
Solvolysis Explained
Essence and Chemical Process
Solvolysis is a chemical reaction where a solute breaks down in a solvent, leading to the cleavage of bonds within the solute molecule. This process is significant in organic chemistry, where it often involves the breakdown of complex molecules into simpler forms, facilitated by the solvent.
- Essence: At its core, solvolysis represents the solvent’s ability to promote chemical reactions by interacting with solute molecules.
- Chemical Process: The process varies depending on the solvent and solute involved but typically results in the substitution or elimination of groups within the solute molecule.
Common Solvents Used
Several solvents are prevalent in solvolysis reactions, each offering unique properties that influence the reaction’s course.
- Water (H₂O): Often used in hydrolysis, where water acts as the solvent.
- Alcohols (R-OH): Common in reactions where an alcohol group facilitates the breaking of bonds.
- Acetic Acid: Utilized in acyl substitution reactions due to its solvent properties.
Aminolysis Uncovered
Core Definition
Aminolysis is a specific type of chemical reaction where an amine group replaces a leaving group in a molecule. This reaction is crucial for introducing nitrogen into organic compounds, enabling the synthesis of a wide range of chemical products.
- Essence: Aminolysis centers on the interaction between amines and organic compounds, resulting in the introduction of amine functionalities.
- Chemical Process: The process involves the nucleophilic attack of an amine on a carbon atom, leading to the substitution of the leaving group.
Typical Amines Involved
The type of amine can significantly affect the aminolysis reaction, with primary, secondary, and tertiary amines all playing roles.
- Primary Amines (RNH₂): Often result in direct substitution.
- Secondary Amines (R₂NH): Can lead to more complex reaction pathways.
- Tertiary Amines (R₃N): Typically do not undergo aminolysis due to steric hindrance.
Mechanism Comparison
Solvolysis Mechanism
Step-by-Step Process
Solvolysis mechanisms can vary, but a general step-by-step process might look like this:
- Solvent Activation: The solvent molecules surround the solute.
- Bond Cleavage: The solvent interacts with the solute, breaking bonds.
- Substitution/Elimination: A new bond forms or an atom/group is eliminated.
Energy Considerations
- Activation Energy: The energy required to initiate the reaction.
- Transition State: The high-energy state during the reaction process.
Aminolysis Mechanism
Detailed Reaction Pathway
The pathway for aminolysis involves several key steps:
- Nucleophilic Attack: The amine attacks the carbon atom bonded to the leaving group.
- Formation of Intermediate: A temporary complex forms during the substitution.
- Product Formation: The leaving group is replaced by the amine, forming the final product.
Role of Amines
Amines act as nucleophiles, donating a pair of electrons to form a new bond with the carbon atom. The nature of the amine (primary, secondary, tertiary) can significantly influence the reaction’s outcome.
Factors Influencing Reactions
Impact of Solvent
Polarity Effects
- Polarity: Affects the solubility of reactants and the reaction’s rate.
- Solvent-Solute Interactions: Crucial for stabilizing the transition state and facilitating the reaction.
Temperature’s Role
Reaction Rate Variation
- Temperature Increase: Generally accelerates the reaction rate.
- Arrhenius Equation: Describes how reaction rates increase with temperature.
Applications and Examples
Solvolysis in Industry
Pharmaceutical Synthesis
Solvolysis is used to synthesize and modify pharmaceutical compounds, enabling the creation of drugs with specific properties.
Environmental Implications
- Degradation: Solvolysis can help degrade harmful substances, reducing environmental pollution.
Aminolysis Applications
Polymer Modification
Aminolysis plays a key role in modifying polymers, enhancing their properties for various applications.
Biochemical Synthesis
- Nitrogen Incorporation: Aminolysis is essential for synthesizing biochemical compounds that require nitrogen functionalities.

Reaction Conditions
Optimal Conditions for Solvolysis
Solvent Selection
Choosing the right solvent is crucial for solvolysis. The solvent must dissolve the reactants well and stabilize the transition state. Water and alcohols are common choices due to their polarity and ability to form hydrogen bonds, which can stabilize charged intermediates in reactions.
Temperature and Pressure
Temperature and pressure significantly affect solvolysis. Higher temperatures usually increase reaction rates by providing more energy to surpass the activation energy barrier. However, optimal pressure conditions can vary, often depending on the solvent’s boiling point and the reaction’s specific needs.
Favorable Aminolysis Conditions
Amine Selection
The choice of amine in aminolysis affects the reaction’s outcome. Primary amines are often preferred for their reactivity and ability to form stable products. The steric and electronic properties of the amine also influence the reaction’s rate and selectivity.
Environmental Factors
Environmental conditions such as pH and solvent polarity play a significant role in aminolysis. For instance, a neutral or slightly basic environment can enhance the nucleophilicity of amines, facilitating the reaction.
Product Analysis
Identifying Reaction Products
Techniques and Tools:
- Chromatography (HPLC, GC): Separates and identifies components in a mixture.
- Mass Spectrometry: Determines molecular weights and structures.
- NMR Spectroscopy: Provides detailed information about molecular structure.
Analysis of By-products
Understanding by-products is essential for assessing reaction efficiency and safety. Techniques like GC-MS (Gas Chromatography-Mass Spectrometry) can identify and quantify these compounds.
Reaction Efficiency
Yield Calculations
Yield is a key indicator of reaction efficiency. It’s calculated as the ratio of the actual amount of product obtained to the theoretical maximum possible, expressed as a percentage.
Purity Assessment
Purity affects the quality and usability of the final product. Techniques such as HPLC (High-Performance Liquid Chromatography) and TLC (Thin Layer Chromatography) are vital for assessing purity levels.
Advantages and Limitations
Solvolysis Benefits
Versatility in Applications
Solvolysis is versatile, applicable in synthesizing a wide range of chemical products, from pharmaceuticals to plastics.
Safety and Environmental Impact
Many solvolysis reactions occur under relatively mild conditions, reducing the need for hazardous reagents and minimizing waste.
Aminolysis Advantages
Specificity and Selectivity
Aminolysis offers high specificity, allowing for targeted synthesis of complex molecules with desired functionalities.
Bio-compatibility
Many aminolysis products are bio-compatible, making them suitable for pharmaceutical and biomedical applications.
Challenges and Solutions
Reaction Control
Controlling reaction conditions to achieve desired outcomes can be challenging. Solutions include:
- Precise temperature and pressure control
- Careful selection of reagents and solvents
Minimizing Side Reactions
Side reactions can lead to undesired products. Strategies to minimize these include:
- Optimizing reaction conditions (pH, temperature)
- Using selective catalysts
Future Directions
Research Trends
Innovative Solvents
Research is focusing on developing eco-friendly solvents that enhance reaction efficiency while reducing environmental impact.
Catalyst Development
Catalysts that can increase reaction rates and selectivity are under intense study, including enzyme mimics and metal-organic frameworks.
Sustainable Practices
Green Chemistry Initiatives
Green chemistry aims to make chemical processes more sustainable by minimizing waste and energy consumption.
Waste Reduction Techniques
Techniques such as recycling solvents and using renewable resources as raw materials are gaining traction.
FAQs
What Is Solvolysis?
Solvolysis is a chemical reaction in which a solute molecule reacts with a solvent, causing the cleavage of a covalent bond in the solute. This process often leads to the formation of new products, where the solvent plays a crucial role in determining the reaction pathway and the nature of the resulting compounds. Solvolysis reactions are integral in various industrial and pharmaceutical processes, offering pathways to synthesize or modify organic compounds.
How Does Aminolysis Differ from Solvolysis?
Aminolysis differs from solvolysis primarily in the reactants involved and the type of chemical bond formation. In aminolysis, an amine group replaces a leaving group in a molecule, introducing nitrogen-containing functionalities. This reaction is pivotal in producing polymers, pharmaceuticals, and dyes. Unlike solvolysis, which often involves water or alcohols as solvents, aminolysis specifically requires an amine to proceed, affecting the reaction’s outcome and applications.
What Are the Applications of Solvolysis and Aminolysis?
Solvolysis and aminolysis find extensive applications in the pharmaceutical, polymer, and environmental sectors. Solvolysis reactions are key in drug synthesis, where they help modify or break down organic compounds. Aminolysis is fundamental in polymer science, enabling the modification of polymers for various uses, including medical devices and biodegradable materials. Both reactions contribute to advancing sustainable chemistry practices and developing new materials.
Conclusion
The exploration of solvolysis and aminolysis opens a window into the complex world of chemical reactions, highlighting their distinct mechanisms, applications, and impacts on science and industry. These processes not only facilitate the synthesis of a broad array of organic compounds but also embody the principles of innovation and sustainability in chemistry.
Understanding the differences and similarities between solvolysis and aminolysis enriches our knowledge of chemical dynamics, guiding researchers and industry professionals in developing more efficient and environmentally friendly synthesis methods. The continuous study and application of these reactions promise to advance our capabilities in material science, pharmaceuticals, and beyond, marking an exciting frontier in chemical research and industrial application.

