Silicon and carbon, two pivotal elements in the periodic table, form the backbone of modern science and technology. While both elements share a place in the same group, their unique properties and roles in the natural world and technological applications set them apart. This distinction not only underscores the diversity of elements but also highlights the complexity of their interactions in various domains.
Silicon and carbon differ primarily in their chemical and physical properties, leading to their diverse roles across different fields. Silicon, a metalloid, is crucial for its semiconductor properties, forming the foundation of modern electronics and computer technology. Carbon, on the other hand, is the key element of all known life, with its ability to form a wide variety of compounds, including the DNA and proteins that make up living organisms.
In the realm of nature and technology, silicon and carbon carve distinct paths. Carbon’s unparalleled versatility in forming organic compounds contrasts with silicon’s structural and electronic utility. This dichotomy not only fascinates scientists but also engineers who leverage these elements for innovations ranging from advanced materials to the next generation of computing technologies. The exploration of these elements reveals the essence of their significance, shedding light on their irreplaceable roles in both the natural world and human-made inventions.

Elemental Basics
Silicon Overview
Atomic Structure
Silicon, symbolized as Si, stands as the 14th element on the periodic table. It possesses a crystal lattice structure similar to that of diamond, making it exceptionally strong and hard. At its core, silicon has 14 electrons, with a configuration that allows it to readily form bonds with other elements.
Physical Properties
Silicon exhibits intriguing physical properties. It’s a semi-metal or metalloid, meaning it partially conducts electricity. This property is pivotal for its use in semiconductors. Silicon is also resistant to high temperatures and oxidation, which makes it suitable for use in a wide range of environments.
Common Compounds and Uses
Silicon forms various compounds, notably silicon dioxide (SiO2), found in sand and quartz. Its compounds are used in:
- Electronics: as a primary material for semiconductor chips.
- Construction: glass and concrete rely on silicon compounds.
- Medical devices: due to its biocompatibility.
Carbon Overview
Atomic Structure
Carbon, with the symbol C, is the 6th element on the periodic table. Its atomic structure is notable for the four valence electrons that allow it to form strong covalent bonds with a variety of other elements. This bonding capacity is the foundation of organic chemistry.
Physical Properties
Carbon’s physical forms, or allotropes, include diamond, graphite, and more recently discovered forms like fullerenes and nanotubes. Diamond is the hardest known natural material, while graphite is soft and conductive. These allotropes highlight carbon’s versatility.
Role in Organic Chemistry
Carbon is the cornerstone of organic chemistry, forming the backbone of biological molecules such as proteins, nucleic acids, and carbohydrates. Its ability to create long chains and rings through covalent bonding makes it unparalleled in forming complex and diverse molecules.
Chemical Behavior
Bonding Patterns
Covalent Bonding in Carbon
Carbon’s four valence electrons enable it to form stable covalent bonds with a wide array of elements. This capacity underpins the vast diversity of organic compounds, from simple molecules like methane (CH4) to complex polymers and biological macromolecules.
Silicon’s Bonding Versatility
While silicon can also form covalent bonds, it typically bonds with oxygen to form silicates and silicon dioxide. Its tetrahedral bonding pattern is crucial in materials like silicones and various ceramics, highlighting its versatility beyond electronics.
Reactivity
Reactivity with Other Elements
Carbon’s reactivity varies widely across its allotropes; diamond is nearly inert, while graphite is more reactive, particularly at high temperatures or with strong oxidizers. Silicon’s reactivity, however, is generally moderate, being less reactive than carbon in its elemental form but highly reactive when combined with oxygen.
Stability in Various Environments
Carbon compounds can be incredibly stable, as seen in fossil fuels and diamonds. Similarly, silicon compounds, especially silicones, exhibit remarkable stability across a broad range of temperatures and chemical conditions, making them valuable in harsh environments.
Role in Nature
Carbon’s Biological Importance
Basis of Life
Carbon is the foundation of all known life forms on Earth. Its unparalleled ability to form diverse organic compounds is critical for the structure and function of cells, the energy transfer in living organisms, and the genetic information encoded in DNA.
Carbon Cycle in Nature
The carbon cycle is a fundamental ecological process that regulates Earth’s climate and the energy flow in the biosphere. It involves the exchange of carbon among the Earth’s atmosphere, oceans, ecosystem, and geosphere, crucial for sustaining life and maintaining the planet’s balance.
Silicon in the Natural World
Abundance in the Earth’s Crust
Silicon is the second most abundant element in the Earth’s crust, primarily as silicon dioxide in sand and minerals. Its abundance is crucial for the planet’s geology, influencing the formation of rocks and continental structures.
Role in Geology and Plant Life
In geology, silicon compounds form minerals and rocks that shape the Earth’s surface. In biology, silicon is beneficial for some plants, providing structural support and protection against pests. This dual role highlights silicon’s importance beyond human-made technologies, playing a significant part in the natural world.
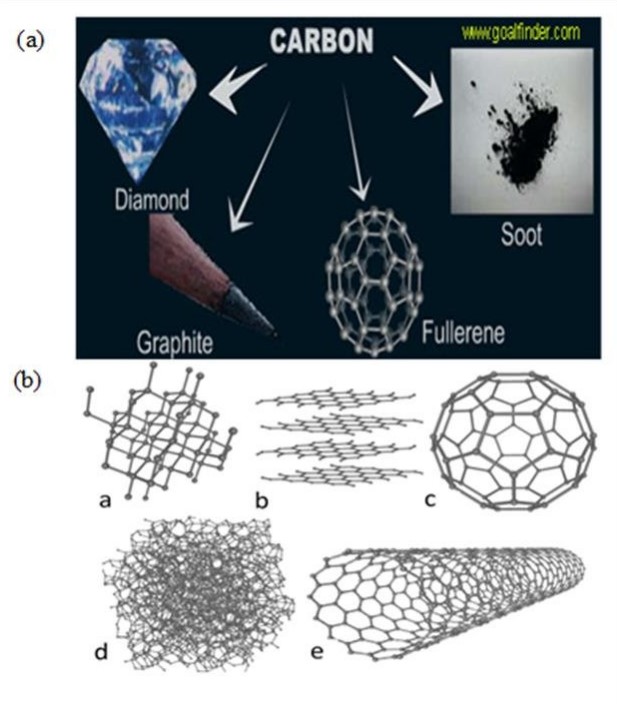
Technological Applications
Silicon in Technology
Semiconductors and Electronics
Silicon stands at the heart of the semiconductor industry. Its ability to act as a semiconductor, meaning it can conduct electricity under some conditions but not others, makes it ideal for chips and circuits. This property is critical in:
- Computers: Silicon chips process information.
- Smartphones: They’re the brain of the device.
- Solar Panels: Silicon converts sunlight into electricity.
Silicones in Materials Science
Silicones, derived from silicon, are used in a variety of products due to their flexibility, heat resistance, and water repellency. Key applications include:
- Medical devices: Safe for use in the human body.
- Cookware: Heat-resistant and non-stick surfaces.
- Sealants and adhesives: Durable and weather-resistant.
Carbon’s Diverse Uses
Carbon Materials in Technology
Carbon’s allotropes, such as graphite and diamond, have unique properties that are exploited in technology. Graphite is used in batteries and lubricants, while diamond’s hardness makes it ideal for cutting tools and abrasives.
Organic Chemistry and Pharmaceuticals
Carbon is the backbone of organic chemistry, leading to the development of numerous pharmaceuticals and synthetic materials. Carbon compounds are essential in:
- Drugs: From painkillers to antibiotics.
- Plastics: Diverse materials for countless uses.
Environmental Impact
Carbon Footprint
Greenhouse Gases and Global Warming
Carbon dioxide (CO2), a greenhouse gas, is a major contributor to global warming. Emissions from burning fossil fuels, deforestation, and other human activities increase the greenhouse effect, leading to climate change.
Efforts to Reduce Carbon Emissions
Reducing carbon emissions is crucial. Strategies include:
- Renewable energy: Solar and wind power reduce reliance on fossil fuels.
- Energy efficiency: Less energy use means lower emissions.
- Carbon capture: Technologies that remove CO2 from the air.
Silicon’s Environmental Considerations
Mining and Processing Impact
The extraction and processing of silicon from the earth can have environmental impacts, including habitat destruction and water pollution. Responsible mining practices and regulations are necessary to minimize these effects.
Disposal and Recycling Challenges
Silicon-based products, especially electronics, present challenges in disposal and recycling. E-waste is a growing problem, requiring proper recycling facilities and methods to recover valuable materials and reduce environmental harm.
Future Perspectives
Innovations in Carbon Use
Advanced Materials and Carbon Nanotechnology
The future of carbon in technology looks bright, with research into carbon nanotubes and graphene. These materials promise:
- Stronger: Than steel but much lighter.
- Conductive: Improved electrical and thermal conductivity.
- Flexible: Potential for flexible electronics.
Silicon Breakthroughs
Next-Generation Semiconductors
Research is pushing the boundaries of silicon in semiconductors, aiming for smaller, faster, and more efficient devices. This includes exploring new materials to complement or replace silicon in certain applications.
Solar Energy Advancements
Silicon remains central to solar energy technology. Improvements in silicon solar cells aim to increase their efficiency and reduce manufacturing costs, making solar power more accessible and sustainable.
Frequently Asked Questions
Why is silicon used in electronics?
Silicon’s use in electronics is attributed to its semiconductor properties, which allow it to efficiently conduct electricity under certain conditions. This unique capability makes silicon an ideal material for creating integrated circuits and transistors, which are the building blocks of all modern electronic devices, from smartphones to computers and beyond.
How does carbon form the basis of life?
Carbon forms the basis of life due to its unparalleled ability to form stable, long chains of molecules and complex structures like rings. This versatility enables carbon to be the primary component of proteins, carbohydrates, fats, and nucleic acids, making it indispensable for life as we know it.
What are the environmental impacts of silicon?
The environmental impact of silicon primarily arises from its mining and processing, which can lead to land degradation, water pollution, and habitat destruction. However, silicon’s role in solar panels and other renewable energy technologies also presents a positive environmental aspect by contributing to the reduction of greenhouse gas emissions.
Can carbon and silicon form compounds together?
Yes, carbon and silicon can form compounds together, known as organosilicon compounds. These compounds are significant in both organic chemistry and materials science, offering properties that are beneficial in various applications such as lubricants, adhesives, and pharmaceuticals, showcasing the versatility of combining these two elements.
Conclusion
Silicon and carbon, each with their unique properties and applications, illustrate the richness of the natural world and human ingenuity. Their differences illuminate the vast possibilities that arise from the basic building blocks of the universe, driving innovation and discovery across countless fields. As we continue to explore and understand these elements, their significance in both nature and technology will undoubtedly remain a subject of fascination and study.
The exploration of silicon and carbon not only enhances our scientific knowledge but also paves the way for future advancements. By harnessing their distinct characteristics, we can continue to push the boundaries of technology, medicine, and environmental sustainability. Their story is a testament to the power of elemental diversity, driving the progress of humanity forward in an ever-changing world.
