Organic chemistry, a branch of science dedicated to the study of the structure, properties, and reactions of organic compounds and materials, continually highlights key compounds and reagents crucial to scientific advancement. Among these, Schiff bases and Schiffs reagent stand out due to their unique applications and functionalities. Both play significant roles in various chemical syntheses and analytical procedures, but they are often confused due to their similar nomenclature.
Schiff bases are compounds typically formed by the condensation of primary amines with aldehydes to yield a compound with a characteristic imine or azomethine group. On the other hand, Schiffs reagent, a specific formulation involving fuchsin and hydrochloric acid, is primarily used for detecting aldehydes in histological samples. These distinctions not only affect their chemical behavior but also their application in different scientific fields.
While Schiff bases are integral in the synthesis of fine chemicals, pharmaceuticals, and dyes, Schiffs reagent plays a crucial role in laboratory diagnostics, staining biological specimens to indicate the presence of aldehyde functionalities. This difference in utility underscores the importance of clearly understanding each compound’s chemical nature and applications in various domains.
Schiff Base Explained
Definition and Formation
A Schiff base refers to a specific type of organic compound characterized by the presence of an imine or azomethine group, which consists of a double bond between a nitrogen atom and a carbon atom. This functional group is pivotal due to its versatility in chemical synthesis and reactivity.
Basic Chemical Structure
The basic structure of a Schiff base is R1R2C=NR3, where R1 and R2 can be hydrogen or alkyl groups, and R3 is usually an alkyl or aryl group. This structure forms the backbone of many more complex compounds in organic chemistry.
Formation Process from Aldehydes and Amines
The formation of Schiff bases is typically achieved through a condensation reaction between an aldehyde and a primary amine. Here are the simplified steps:
- Aldehyde Selection: An aldehyde (R-CHO) is chosen based on the desired properties of the final Schiff base.
- Amine Addition: A primary amine (H2N-R’) is reacted with the aldehyde under controlled conditions.
- Water Removal: As the reaction proceeds, water (H2O) is formed as a byproduct and is continuously removed to drive the reaction forward, forming the imine link, R-CH=N-R’.
Properties
Physical and Chemical Properties
Schiff bases are known for their moderate to good stability under normal conditions but can be sensitive to hydrolysis back into their constituent amine and aldehyde under acidic or very moist conditions. They are generally solid at room temperature and can show a range of colors from colorless to deep yellows and reds, depending on the substituents attached to the imine function.
Applications
Uses in Various Chemical Processes
Schiff bases are extensively utilized in organic synthesis, serving as ligands in coordination chemistry, catalysts in various chemical reactions, and intermediates in the synthesis of more complex organic molecules.
Examples in Industrial Applications
- Dye Synthesis: Schiff bases act as intermediates in the synthesis of azo dyes, which are widely used in textile industries.
- Pharmaceuticals: They are used in the formulation of various drugs, including antifungal, antibacterial, and anticancer agents due to their ability to interact with biologically relevant metals.
- Catalysis: In industrial processes, Schiff bases facilitate certain types of chemical reactions, enhancing reaction speeds and selectivity.
Schiffs Reagent Explored
Composition
Chemical Composition and Preparation
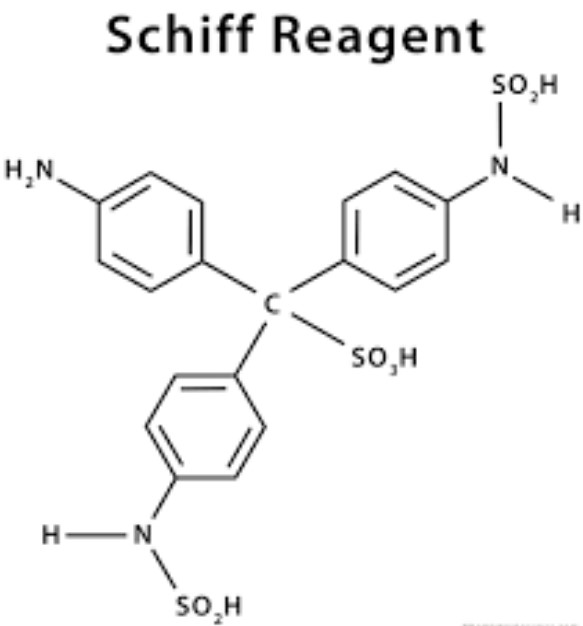
Schiffs reagent is prepared by treating a solution of fuchsin with sulfurous acid, which effectively removes color from the fuchsin solution. When aldehydes are present, they react with the reagent, restoring the pink color, which is indicative of their presence.
Detection Mechanism
Role in Detecting Aldehydes
The primary role of Schiffs reagent is in the detection of aldehydes in biological and chemical samples. The process involves:
- Sample Preparation: The test sample is prepared by ensuring it is in a suitable medium for the reagent.
- Reagent Application: Schiffs reagent is applied to the prepared sample.
- Color Change Observation: A positive test is indicated by a change in color of the sample area where the reagent was applied, signifying the presence of aldehydes.
Utility
Applications in Laboratory Settings
Schiffs reagent is invaluable in histological laboratory settings for:
- Staining Tissues: Particularly useful in highlighting mucopolysaccharides and glycoproteins in animal tissues.
- Pathology Tests: It helps in diagnosing diseases by staining specific cellular components that contain aldehydes.
Comparative Analysis
Chemical Differences
Structural Distinctions Between Schiff Base and Schiffs Reagent
Schiff bases and Schiffs reagent, while related in name, exhibit distinct structural characteristics. Schiff bases are formed through a simple condensation reaction between an aldehyde and an amine, resulting in a compound that features an imine group, characterized by a C=N bond. This structural motif allows for a variety of R-groups to be attached, affecting the physical and chemical properties of the resulting Schiff base.
In contrast, Schiffs reagent is not a single compound but a solution primarily composed of decolorized fuchsin and sulfurous acid. It does not contain an imine group but is used to react with aldehyde groups in other molecules, thereby serving a different chemical purpose.
Functional Contrast
Different Roles in Chemical Reactions and Processes
The functional capabilities of Schiff bases and Schiffs reagent diverge significantly due to their structural differences. Schiff bases, with their versatile imine linkage, are used broadly in organic synthesis. They can act as ligands in coordination chemistry, forming complexes with metals, which are useful in catalysis and material science.
On the other hand, Schiffs reagent is primarily used as a diagnostic tool. It reacts with aldehydes to form a magenta-colored compound, which is used extensively in histological staining to identify aldehyde groups in tissues, serving an essential role in medical diagnostics and research.
Practical Examples
In Laboratory
Common Tests and Experiments Using Schiffs Reagent
Schiffs reagent is a staple in many laboratory settings due to its specificity for aldehydes. Some common applications include:
- Histological Staining: Used to stain aldehyde groups in tissues, aiding in the identification of cellular components during medical examinations.
- Formaldehyde Detection: Schiffs reagent is employed in industrial settings to test for the presence of formaldehyde, a common indoor air pollutant.
In Industry
Industrial Synthesis Involving Schiff Bases
Schiff bases are integral to various industrial applications due to their chemical properties. Examples include:
- Catalysis: Schiff bases are used as catalysts in the synthesis of fine chemicals, enhancing the efficiency and specificity of chemical reactions.
- Dye Manufacturing: The vibrant colors and ease of modification make Schiff bases ideal intermediates in dye production, particularly in textile manufacturing.
- Pharmaceuticals: They are employed in the creation of drug molecules, particularly those involving complexation with metals, useful in treatments ranging from anticancer to antibacterial therapies.
FAQ Section
What are Schiff bases?
Schiff bases are a class of organic compounds characterized by a functional group containing a double bond between a nitrogen and a carbon atom. This group is typically formed by the reaction between an aldehyde and a primary amine. These bases are versatile intermediates used in the synthesis of many other chemical compounds, including dyes and pharmaceuticals.
How is Schiffs reagent prepared?
Schiffs reagent is prepared by decolorizing a solution of basic fuchsin with sulfurous acid, which is then used in the presence of a weak acid. This preparation is used for the specific detection of aldehyde functional groups in tissue samples, making it invaluable in histological diagnostics.
What distinguishes Schiff bases from Schiffs reagent?
While Schiff bases are formed from aldehydes and amines and feature in various synthetic applications, Schiffs reagent is specifically formulated for the detection of aldehydes. This reagent is not a Schiff base but a diagnostic tool used in biological staining, highlighting the functional differences between the two.
Can Schiff bases be used in medical applications?
Yes, Schiff bases are utilized in various medical applications, including as antibacterial, antifungal, and anticancer agents. Their ability to easily form complexes with metals can be harnessed to create compounds that are biologically active and useful in therapeutic treatments.
Conclusion
Understanding the distinct characteristics and applications of Schiff bases and Schiffs reagent enriches the repertoire of tools available to chemists and researchers in the field of organic chemistry. This knowledge not only facilitates the appropriate use of each compound in its respective domain but also highlights the breadth of chemistry as a science of endless possibilities and discoveries.
The nuances between these compounds underline the importance of accurate chemical education and the need to distinguish closely related yet functionally different substances. With continued research and educational efforts, the applications and understanding of such compounds will undoubtedly expand, contributing further to scientific and technological advancements.

