Organic chemistry plays a pivotal role in the development of various industrial and pharmaceutical products. One of its core components is the understanding of how different rules predict the outcome of elimination reactions. Among these, the Saytzeff and Hofmann rules are crucial for predicting the major products of alkene formation reactions. These rules help chemists manipulate chemical reactions to achieve desired results, highlighting their significance in synthetic chemistry.
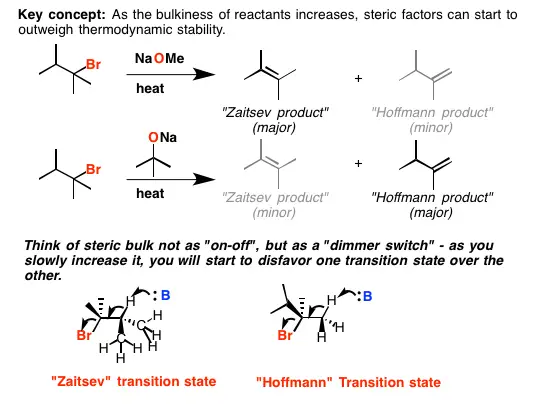
The Saytzeff rule predicts that during an elimination reaction, the more substituted alkene will be the major product. In contrast, the Hofmann rule suggests that the least substituted alkene predominates when certain bulky bases are used. This distinction is fundamental in organic synthesis, where the choice between these two pathways can determine the efficiency and utility of the synthesis.
These rules not only guide the synthesis of complex molecules but also serve as educational foundations in organic chemistry curriculums worldwide. Their application stretches across various fields, from medicinal chemistry to material science, making them essential tools for any chemist.
Historical Context
The journey of understanding how chemical reactions behave under different conditions has profoundly shaped modern organic chemistry. Two pivotal rules in this journey are the Saytzeff rule and the Hofmann rule. These rules provide chemists with guidelines to predict the products of elimination reactions, crucial for synthetic strategies.
Discovery of Saytzeff Rule
The Saytzeff rule was first articulated by Russian chemist Alexander Saytzeff in 1875. While studying the dehydration of secondary and tertiary alcohols, Saytzeff observed that the elimination reaction typically favored the formation of the most substituted alkene. This observation was groundbreaking because it introduced a predictable pattern to what had been thought to be unpredictable organic reactions. His work primarily focused on understanding the behavior of different alkenes produced during the reactions and established a foundational rule still used over a century later.
Discovery of Hofmann Rule
Contrasting the Saytzeff rule, the Hofmann rule originated from the observations made by German chemist August Wilhelm von Hofmann in the 1850s. Hofmann was studying the reactions involving amine derivatives when he found that under certain conditions, particularly when bulky bases are used, the least substituted alkene is favored. This was particularly significant because it offered a new dimension to the understanding of steric effects in chemical reactions.
Evolution of Understanding
The initial discoveries by Saytzeff and Hofmann sparked a series of investigations and debates among chemists about the nature and dynamics of elimination reactions. Over time, the understanding of these rules has been refined by experimental data and theoretical advancements. The evolution has not only validated the initial observations but also expanded the application of these rules in complex organic synthesis.
Saytzeff Rule
Definition and Basics
The Saytzeff rule states that in an elimination reaction, the most substituted product will be the most stable and, therefore, the most favored. This rule is applicable primarily in E1 and E2 elimination reactions where the leaving group and a hydrogen atom are removed from adjacent carbon atoms, leading to alkene formation.
Mechanism of Action
The mechanism underlying the Saytzeff rule involves the formation of a carbocation intermediate in E1 reactions or the transition state in E2 reactions. The stability of these species is enhanced by the presence of alkyl groups that provide electron-donating effects through hyperconjugation and inductive effects. Thus, the more substituted the alkene, the more stable it is, and hence more likely to form.
Common Applications
- Synthesis of Alkenes: Widely used in the laboratory synthesis of alkenes from alcohols and halides.
- Petroleum Refining: Plays a role in the cracking processes where large hydrocarbons are broken down into smaller, more useful pieces.
Example Reactions
- Dehydration of Alcohols: An alcohol like 2-butanol when dehydrated under acidic conditions typically yields but-2-ene as per Saytzeff’s rule.
- Dehydrohalogenation of Alkyl Halides: Treating 2-bromopentane with a strong base will predominantly give pent-2-ene.
Hofmann Rule
Definition and Basics
The Hofmann rule is often seen as the exception to the Saytzeff rule. It predicts that the least substituted alkene will be the major product when bulky bases are used in the elimination reactions. This rule is critical in scenarios where steric hindrance plays a significant role.
Mechanism of Action
The action of the Hofmann rule is largely governed by the steric effects caused by bulky bases. These bases hinder the approach to the more substituted carbons, making it less favorable to form the more substituted alkene. As a result, the reaction pathway shifts towards the formation of the less substituted alkene.
Common Applications
- Preparation of Less Substituted Alkenes: Useful in synthetic chemistry when a less substituted alkene is needed.
- Analytical Chemistry: Employed to explore steric effects in molecules.
Example Reactions
- Hofmann Elimination: The classic example is the reaction of quaternary ammonium salts, which, upon treatment with silver oxide and water, yields the least substituted alkene.
- Use with Bulky Bases: Using a base like tert-butoxide in the elimination reactions of halides favors the formation of less substituted alkenes.

Key Differences
The Saytzeff and Hofmann rules may appear similar as they both guide the outcome of elimination reactions, but they are distinct in several crucial aspects. Understanding these differences is key to applying each rule effectively in organic synthesis.
Rule Focus
The Saytzeff rule focuses on maximizing the stability of the resulting alkene through substitution. It predicts that the alkene formed will have the greatest number of alkyl groups attached to the double-bonded carbons. In contrast, the Hofmann rule prioritizes steric factors over stability, often leading to the formation of the least substituted, and therefore often less stable, alkene.
Conditions Favored
- Saytzeff Rule: Favors conditions where small, non-bulky bases are used, and the reaction medium does not impose steric hindrance on the reaction pathway.
- Hofmann Rule: Favored in reactions using bulky bases that create significant steric hindrance, influencing the mechanism to favor less substituted alkenes.
Resulting Products
- Saytzeff Rule: Typically results in more substituted alkenes, which are generally more stable due to hyperconjugation and inductive effects.
- Hofmann Rule: Leads to the formation of less substituted alkenes due to steric hindrance preventing the base from approaching the more hindered (substituted) beta carbon.
Chemical Examples
- Saytzeff Rule Example: Dehydration of 2-methyl-2-butanol typically yields 2-methyl-2-butene.
- Hofmann Rule Example: Elimination of N,N-dimethyl-3-iodo-3-methylbutylamine using potassium tert-butoxide in tert-butanol often yields 2-methyl-1-butene.
Practical Implications
The implications of these rules stretch far beyond the confines of theoretical chemistry, influencing various practical aspects of chemical synthesis and industrial applications.
Influence on Synthesis
Chemists often have to decide which elimination method to employ based on the desired product. For instance, if a more stable product is desired, the Saytzeff rule is followed, whereas for less substituted products that might be desirable in certain syntheses, the Hofmann rule is employed.
Industrial Applications
- Saytzeff Rule: Widely used in the design of drugs where stability of the molecular framework is crucial.
- Hofmann Rule: Often utilized in the synthesis of polymers where specific monomer units are required to be less substituted for subsequent chemical reactions.
Academic Relevance
These rules are integral to the curriculum of organic chemistry courses around the world. They serve as excellent examples of how theoretical principles are directly applied in practical scenarios, teaching students both the fundamentals of chemical reactivity and the decision-making process in synthetic pathways.
Comparative Analysis
A deeper understanding emerges when comparing these rules through practical scenarios and examining their strengths and limitations.
Scenario-Based Comparison
Consider the synthesis of an alkene from a halogenated precursor:
- Using a small base like hydroxide ion might follow Saytzeff’s rule to give a more substituted, stable alkene.
- Switching to a bulky base like potassium tert-butoxide could shift the reaction to follow Hofmann’s rule, resulting in a less substituted alkene.
Strengths and Limitations
- Strengths of Saytzeff Rule: Predicts the most stable alkene; widely applicable.
- Strengths of Hofmann Rule: Useful when steric hindrance is a desired feature in synthesis.
- Limitations: Each rule has exceptions and is heavily dependent on the reaction conditions, which can complicate predictions.
Decision Factors in Application
The choice between Saytzeff and Hofmann elimination often hinges on:
- Desired product specification.
- The structure of the starting material.
- The nature of the base used.
Future Prospects
The future of these foundational rules in chemistry appears robust, with ongoing research likely to expand their applicability and precision.
Recent Research Trends
Recent studies focus on developing more selective catalysts and bases that can specifically favor either Saytzeff or Hofmann products under a broader range of conditions, enhancing both rules’ utility.
Innovations and Modifications
Modifications to existing bases and the development of new synthetic strategies continue to refine the predictability and outcomes of elimination reactions, pushing the boundaries of what can be achieved using these rules.
Potential Impact on Industry
The ongoing advancements in understanding and applying the Saytzeff and Hofmann rules have significant implications for pharmaceuticals, agrochemicals, and material sciences. As new methods and materials emerge, these rules will play a crucial role in the development of more effective and efficient synthetic pathways, benefiting multiple industries.
Frequently Asked Questions
What is Saytzeff’s Rule?
Saytzeff’s rule, also known as Zaitsev’s rule, is a principle in organic chemistry that predicts the formation of the more substituted alkene as the major product in elimination reactions. This rule is particularly useful when predicting the outcome of dehydrohalogenation reactions.
How does Hofmann’s Rule differ?
Hofmann’s rule predicts that the least substituted alkene will predominantly form in elimination reactions involving bulky bases. This rule often applies in cases where steric hindrance influences the reaction mechanism, favoring less substituted alkene formation.
When do you apply these rules?
These rules are applied during elimination reactions in organic synthesis. Saytzeff’s rule is generally used with small bases, while Hofmann’s rule is preferred with larger, more bulky bases that affect the reaction’s steric outcomes.
Why are these rules important?
Saytzeff and Hofmann rules help chemists understand and control the products of elimination reactions. By predicting the major product, these rules enable more efficient synthetic routes and help in the design of molecules with specific properties and functions.
Conclusion
The Saytzeff and Hofmann rules are more than just guidelines; they are fundamental principles that shape the course of chemical reactions and synthetic strategies. Their relevance extends beyond the laboratory, influencing the methodologies taught in academic institutions and applied in industrial settings.
As the field of organic chemistry continues to evolve, the importance of understanding these rules remains undiminished. They not only equip chemists with the knowledge to manipulate chemical reactions but also inspire continuous innovation in the synthesis of new and improved materials and drugs.

