Chromatography stands as a cornerstone technique in the separation and analysis of compounds, vital to various scientific and industrial applications. Among its many types, Reverse Phase High-Performance Liquid Chromatography (RP HPLC) and Hydrophobic Interaction Chromatography (HIC) are prominent for their specific utilities in purifying and analyzing molecules. These methodologies, though grounded in the principle of exploiting hydrophobic interactions, differ significantly in their approach and application.
RP HPLC and HIC are distinguished primarily by their use of stationary phases and the nature of their interactions with analytes. RP HPLC relies on nonpolar stationary phases and is used extensively for its versatility in separating a wide range of molecules, including small to medium-sized ones. HIC, on the other hand, utilizes slightly polar stationary phases to retain substances based on their hydrophobicity, making it ideal for separating large biomolecules such as proteins.
The selection between RP HPLC and HIC hinges on the specific requirements of the analysis or purification task at hand. Factors such as the size of the molecules to be separated, their hydrophobicity, and the desired resolution and specificity of the separation process guide the choice of technique. Each method has its unique advantages, with RP HPLC offering broad applicability and HIC providing specialized utility for protein purification, highlighting the importance of understanding these differences for optimal application in scientific research and industrial processes.
RP HPLC Basics
What is RP HPLC?
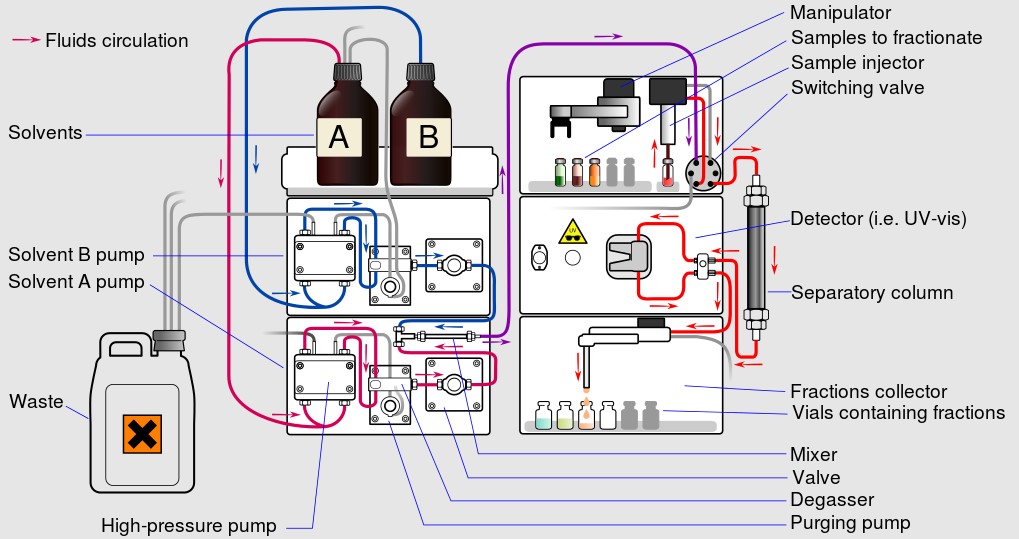
Reverse Phase High-Performance Liquid Chromatography (RP HPLC) stands out as a pivotal analytical technique. Employed across various scientific disciplines, RP HPLC facilitates the separation and identification of compounds within a mixture. This technique leverages a nonpolar stationary phase and a comparatively polar mobile phase, a setup that ensures compounds are separated based on their hydrophobicity.
Principle of Operation
The core principle behind RP HPLC is the interaction between the analyte, the stationary phase, and the mobile phase. Analytes in the mixture interact with both phases; however, their affinity towards the stationary phase, which is typically made of silica particles coated with hydrophobic chains (like C18), dictates the separation process. The more hydrophobic a compound is, the longer it will be retained on the column.
- Mobile phase adjustment allows fine-tuning of the separation process.
- Analytes are detected as they elute from the column based on their retention time.
Key Applications
RP HPLC has a broad application spectrum, serving essential roles in:
- Pharmaceuticals: For purity assessment, drug formulation, and metabolite analysis.
- Food Industry: In contaminant identification and nutritional analysis.
- Environmental Testing: Detecting pollutants in water and soil.
- Research: Facilitating biomolecule studies, including peptides and other small proteins.
HIC Fundamentals
Definition of HIC
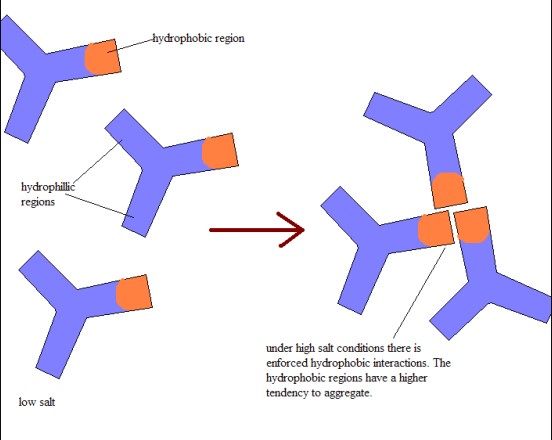
Hydrophobic Interaction Chromatography (HIC) is a technique that differentiates molecules based on their hydrophobicity. It is uniquely suited for the purification of proteins and other large biomolecules, which can form reversible interactions with the hydrophobic media, typically under high-salt conditions.
Operating Principles
HIC operates on the premise that proteins and other biomolecules exhibit hydrophobic surfaces. When exposed to high concentrations of salt in the mobile phase, these hydrophobic sites are prompted to interact with the hydrophobic stationary phase, effectively being retained on the column. The subsequent gradient elution, often involving decreasing salt concentrations, allows for the sequential elution of biomolecules based on their hydrophobicity levels.
- Salt gradients are crucial for modulating the elution process.
- This method is particularly gentle on biomolecules, preserving their native state.
Main Uses
HIC is predominantly used in the biopharmaceutical industry for:
- Purifying antibodies: Especially monoclonal antibodies for therapeutic uses.
- Protein fractionation: Separating proteins from complex biological mixtures.
- Analyzing post-translational modifications: Such as phosphorylation or glycosylation, which can alter hydrophobicity.
Key Differences
Principle and Mechanism
While both RP HPLC and HIC utilize hydrophobic interactions for separation, their underlying mechanisms differ:
- RP HPLC separates molecules based on their overall hydrophobicity, with more hydrophobic compounds being retained longer.
- HIC focuses on the surface hydrophobicity of larger biomolecules, especially under high-salt conditions which enhance these interactions.
Retention Mechanisms in RP HPLC vs HIC
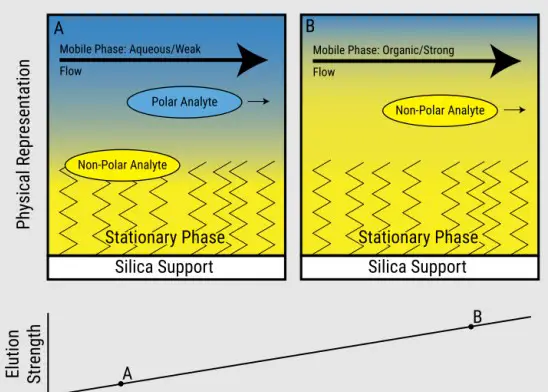
- In RP HPLC, the nonpolar stationary phase and polar mobile phase create a scenario where hydrophobic compounds are retained.
- HIC uses a slightly polar stationary phase in a high-salt buffer, promoting hydrophobic interactions without relying on the polarity contrast used in RP HPLC.
Role of Hydrophobicity
- Hydrophobicity is key in both techniques but is approached differently. RP HPLC assesses overall hydrophobicity, while HIC looks at surface hydrophobic interactions under specific conditions.
Materials and Columns
Types of Stationary Phases
- RP HPLC: Utilizes C18 and other alkyl chain bonded phases.
- HIC: Prefers phenyl or ether linked groups that offer mild hydrophobicity.
Column Selection Criteria
- Particle size, pore size, and bonded phase are critical factors in choosing columns for both techniques, tailored to the separation task.
Mobile Phases
Solvent Systems in RP HPLC
- A mix of water and organic solvents like methanol or acetonitrile adjusts the polarity of the mobile phase in RP HPLC.
Buffer Systems in HIC
- High-salt buffers, such as ammonium sulfate, enhance hydrophobic interactions in HIC, crucial for protein separation.
Sample Preparation
RP HPLC Requirements
- Samples may need to be diluted in compatible solvents or undergo pre-treatment to ensure analytes are in the right form for separation.
HIC Sample Conditions
- Buffer exchange into high-salt solutions is often necessary for HIC, preparing biomolecules for effective hydrophobic interaction.
Sensitivity and Specificity
Analyte Detection in RP HPLC
- RP HPLC is highly sensitive to a wide range of compounds, offering detailed insights into the composition of complex mixtures.
Identifying Molecules with HIC
- HIC excels in the specific separation of large biomolecules, such as proteins, by leveraging subtle differences in hydrophobicity.
Application Scope
Biopolymers Analysis in RP HPLC
- RP HPLC is adept at analyzing biopolymers and small to medium biomolecules, providing quantitative and qualitative data.
Large Biomolecules Separation with HIC
- HIC is the go-to for large biomolecule separation, including therapeutic proteins and antibodies, preserving their native state and functionality.
Choosing Between RP HPLC and HIC
When it comes to separating and purifying chemical compounds, especially in the realm of biochemistry, two prevalent techniques stand out: Reverse Phase High-Performance Liquid Chromatography (RP HPLC) and Hydrophobic Interaction Chromatography (HIC). Each method has its specific strengths and ideal applications. Understanding how to choose between them is crucial for achieving optimal results in the laboratory.
Factors to Consider
The decision to use RP HPLC or HIC hinges on several key factors, each influencing the outcome of the separation process:
- Molecule Size: Large biomolecules often fare better with HIC, which preserves their structure and function.
- Hydrophobicity: RP HPLC is more suited for highly hydrophobic molecules.
- Sensitivity and Resolution Needs: RP HPLC generally offers higher sensitivity and resolution.
- Sample Complexity: The complexity of the sample may dictate the choice based on which method can efficiently handle the mixture.
Compatibility with Sample Types
RP HPLC is exceptionally versatile, making it suitable for a broad range of compounds, from small organic molecules to peptides and proteins. However, its use of organic solvents can denature proteins. HIC, with its gentler separation environment, is better for proteins and large biomolecules that might be sensitive to the conditions used in RP HPLC.
Optimization Tips
Optimizing each chromatography technique ensures high efficiency, resolution, and recovery of the target analytes. Here are some strategies for enhancing both RP HPLC and HIC processes.
For RP HPLC
Enhancing Separation Efficiency
- Gradient Elution: Utilizing a gradient of solvents can improve the separation of compounds with varying hydrophobicities.
- Column Temperature: Adjusting the column temperature can influence the interaction between the analytes and the stationary phase, improving efficiency.
Improving Resolution
- Smaller Particle Size: Using columns packed with smaller particles can enhance resolution but may require higher pressure.
- Column Length: Longer columns offer increased resolution due to a longer separation path.
For HIC
Maximizing Recovery
- Optimize Salt Concentration: Fine-tuning the salt concentration in the mobile phase can improve the binding and elution of proteins, enhancing recovery.
- Column Pre-equilibration: Properly equilibrating the column with the high-salt buffer before sample application is crucial for consistent recovery rates.
Reducing Sample Loss
- Gentle Elution Conditions: Implementing a gradual salt gradient prevents the sudden release of bound proteins, reducing the risk of sample loss.
- Sample Loading: Diluting the sample in a compatible buffer can minimize nonspecific binding and loss.
Common Challenges
Despite the advantages of RP HPLC and HIC, practitioners may encounter challenges that can affect the quality of the separation process. Recognizing and addressing these issues is essential for successful chromatography.
Addressing Issues in RP HPLC
RP HPLC challenges often revolve around sample solubility, peak tailing, and column degradation:
- Improving Sample Solubility: Adjusting the solvent composition or sample pre-treatment can enhance solubility.
- Reducing Peak Tailing: Peak tailing can be mitigated by using buffers at the appropriate pH or adding silanol blockers to the mobile phase.
- Preventing Column Degradation: Proper column maintenance and using guard columns can extend the life of the RP HPLC column.
Overcoming Hurdles in HIC
In HIC, challenges include protein denaturation, low recovery, and buffer compatibility:
- Preventing Protein Denaturation: Ensuring the buffer and salt concentrations do not disrupt protein structure is key.
- Enhancing Recovery: Optimizing the elution gradient and salt type can improve protein recovery.
- Buffer Compatibility: Using buffers that do not interfere with protein binding or elution enhances separation quality.
Frequently Asked Questions
What is RP HPLC?
Reverse Phase High-Performance Liquid Chromatography (RP HPLC) is a widely used analytical technique that separates compounds based on their hydrophobic interactions with a nonpolar stationary phase. It is highly versatile, capable of analyzing a wide range of molecules from small organic compounds to peptides, under high pressure to achieve rapid and high-resolution separations.
How does HIC work?
Hydrophobic Interaction Chromatography (HIC) operates on the principle of retaining analytes based on their hydrophobicity. In HIC, a slightly polar stationary phase is used. Analytes are typically applied in high-salt buffers, which promote hydrophobic interactions between the stationary phase and the analytes, allowing for effective separation of large biomolecules, such as proteins.
When to use RP HPLC over HIC?
RP HPLC is preferred when dealing with a wide variety of molecules, particularly small to medium-sized ones, requiring high resolution and sensitivity. It’s ideal for compounds that are moderately to highly hydrophobic. In contrast, HIC is better suited for separating large biomolecules, like proteins, that may be denatured or lose function under the conditions used in RP HPLC.
Can RP HPLC separate proteins?
Yes, RP HPLC can separate proteins, but it is less commonly used for this purpose compared to HIC. The high organic solvent concentrations required in RP HPLC can denature some proteins, affecting their activity and function. Thus, for protein analysis and purification, HIC or other techniques are often preferred to maintain the native state of proteins.
Conclusion
The nuanced differences between RP HPLC and HIC underscore the importance of selecting the appropriate chromatographic technique tailored to the specific needs of a given analysis or purification task. This selection process not only affects the efficiency and resolution of the separation but also the integrity and functionality of the analytes, especially when dealing with sensitive biomolecules like proteins.
Understanding the operational principles, applications, and limitations of each technique allows researchers and professionals to harness the full potential of chromatography. Whether aiming for the broad applicability and versatility of RP HPLC or the specialized capabilities of HIC for protein purification, a clear grasp of these differences ensures the successful implementation of chromatographic methods in various scientific and industrial contexts.