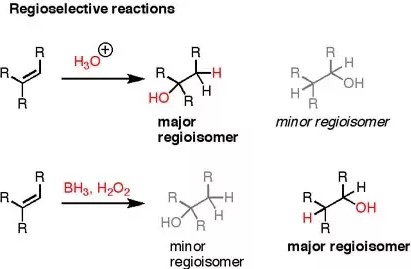
Chemical reactions are the heartbeats of organic synthesis, driving the creation of complex molecules through the manipulation of simpler ones. Among the myriad nuances of these reactions, regioselectivity and stereoselectivity stand out as critical concepts. They guide chemists in predicting and controlling the outcomes of chemical reactions, making them essential for the development of pharmaceuticals, materials, and a wide array of organic compounds.
Regioselectivity and stereoselectivity refer to the preference of a chemical reaction to proceed in one direction over another, leading to the formation of specific products. Regioselectivity concerns the “where” aspect, determining which part of a molecule is most likely to undergo a chemical change. In contrast, stereoselectivity deals with the “how” aspect, specifying the spatial orientation of the atoms or groups in the resultant molecule. Understanding these selectivities is pivotal for chemists aiming to synthesize molecules with desired properties.
In organic synthesis, the distinction between regioselectivity and stereoselectivity is more than academic. It influences how researchers approach the synthesis of molecules, impacts the efficiency of reactions, and plays a crucial role in the development of new drugs and materials. By grasping these concepts, chemists can navigate the complex landscape of organic chemistry with greater precision, optimizing reactions to achieve high yields of the intended products while minimizing unwanted isomers.
Basic Concepts
Chemical Selectivity: Definition and Significance
Chemical selectivity is a fundamental concept in chemistry and organic synthesis. It refers to the ability of a chemical reaction to produce a particular product from among several possible products. Selectivity is crucial because it determines the efficiency and outcome of a reaction, impacting the yield of the desired product and minimizing unwanted byproducts.
Regioselectivity Explained
Basic Definition
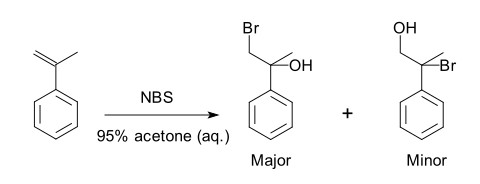
Regioselectivity concerns the preference of a chemical reaction to occur at one specific location or direction on a molecule rather than another. This selectivity dictates where a reaction takes place on the molecular framework, guiding the formation of specific isomers.
Role in Organic Reactions
In organic reactions, regioselectivity is essential for constructing molecules with precise structural characteristics. It impacts how chemists design syntheses, especially when targeting molecules with complex functionalities. The control of regioselectivity enables the efficient creation of compounds with desired properties, a necessity in pharmaceutical development, material science, and various fields of research and industry.
Stereoselectivity Unpacked
Basic Definition
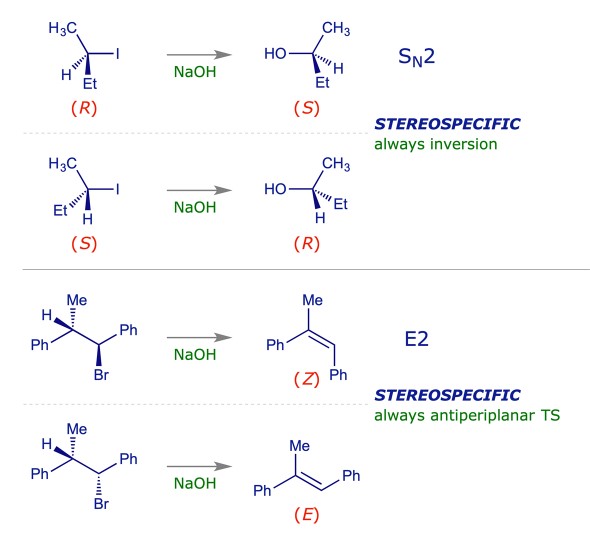
Stereoselectivity involves the preference of a chemical reaction to yield a specific spatial arrangement of atoms or groups in a molecule, leading to the formation of stereoisomers. It is about the ‘how’—the orientation of the resulting molecule.
Importance in Molecular Structure
The stereochemistry of a molecule can dramatically influence its physical, chemical, and biological properties. Stereoselectivity is therefore critical in the synthesis of bioactive compounds, where the spatial arrangement of atoms can determine the effectiveness of a drug, its interaction with biological receptors, and its overall bioavailability.
Key Differences
Fundamental Distinctions
Regioselectivity and stereoselectivity, while both concerned with the selectivity of chemical reactions, differ fundamentally in their focus. Regioselectivity deals with the location of a reaction on a molecule, whereas stereoselectivity is about the orientation of atoms in the product. This distinction is crucial for understanding how each type of selectivity influences the outcome of a chemical reaction.
Comparison of Definitions
- Regioselectivity: Determines the specific site on a molecule where a reaction occurs.
- Stereoselectivity: Dictates the spatial arrangement of atoms or groups in the product.
Reaction Outcomes
The impact on product formation varies significantly between regioselective and stereoselective reactions. Regioselective reactions can lead to different structural isomers, changing the molecule’s functional properties. In contrast, stereoselective reactions produce isomers with different spatial orientations, affecting the molecule’s interaction with its environment and other molecules.
Examples in Organic Chemistry
Regioselective Reactions
- Addition to Alkenes: The Markovnikov addition of hydrogen halides to alkenes is a classic example, where the halide attaches to the more substituted carbon atom.
- Directed Metalation: Certain directing groups influence the site of metalation in aromatic compounds, guiding subsequent reactions to specific positions.
Stereoselective Reactions
- Asymmetric Hydrogenation: Catalytic processes that produce one enantiomer preferentially over another, crucial for synthesizing chiral drugs.
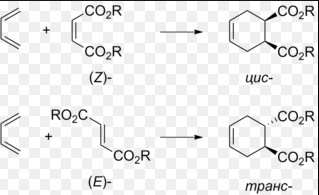
- Diels-Alder Reaction: Cycloadditions where the stereochemistry of the diene and dienophile leads to specific adducts.
Factors Influencing Selectivity
Electronic Effects
Electronic effects significantly influence regioselectivity, directing the course of a reaction based on the electronic distribution within a molecule. Electrophilic additions to unsaturated systems, for example, are swayed by the electronic nature of substituents, favoring reactions at positions that stabilize the developing charge.
Steric Factors
Steric hindrance plays a pivotal role in stereoselectivity, as bulky groups can prevent certain orientations of reactants or products. This physical blocking influences the path a reaction takes, often leading to the formation of the most sterically accessible product.
Catalysts and Conditions
The choice of catalysts and reaction conditions can drastically affect both regioselectivity and stereoselectivity. The use of chiral catalysts, for example, can induce a reaction to favor the formation of one enantiomer over another. Similarly, temperature, solvent, and the presence of directing groups can modify the course of reactions, steering them towards desired outcomes.
- Temperature: Influences the kinetic control of a reaction, which can dictate selectivity.
- Solvent: The polarity and properties of the solvent can affect the reactivity of intermediates, altering selectivity.
- Catalysts: Specific catalysts can favor certain pathways, enhancing the selectivity for one product over another.
Applications in Synthesis
Pharmaceutical Synthesis
Importance of Selectivity
In the world of pharmaceutical synthesis, selectivity is not just important; it’s critical. The creation of drugs that can effectively target specific physiological pathways without causing adverse side effects hinges on the ability to synthesize molecules with precise structural and stereochemical properties. The right or wrong spatial arrangement of atoms in a drug molecule can mean the difference between a lifesaving treatment and an ineffective or potentially harmful compound.
Regioselectivity and stereoselectivity in drug synthesis ensure that the active ingredient possesses the optimal configuration for interacting with biological targets, such as enzymes, receptors, and DNA. This precision drives the efficacy of drugs, affecting their binding affinity, metabolism, and overall therapeutic value. As such, the pharmaceutical industry invests heavily in research and technology to control and predict these selectivities during drug development.
Material Science
Selectivity in Polymerization
In material science, particularly in polymer synthesis, selectivity plays a pivotal role in defining the properties of polymers. The arrangement of monomer units (regioselectivity) and the configuration of stereocenters (stereoselectivity) in a polymer chain can drastically influence its physical properties, such as strength, flexibility, thermal stability, and biodegradability.
- Regioselective polymerization can dictate how different monomer units arrange themselves within a polymer, affecting its functionality and application.
- Stereoselective polymerization controls the spatial arrangement of atoms in the polymer backbone, which can impact the material’s crystallinity and mechanical properties.
The ability to tailor these properties through controlled polymerization techniques has led to the development of advanced materials with specific characteristics for use in a wide range of applications, from medical devices and biodegradable plastics to high-performance fibers and electronics.
Challenges and Solutions
Predicting Selectivity
Computational Methods and Tools
Predicting the outcome of chemical reactions with respect to regioselectivity and stereoselectivity remains a significant challenge. However, advancements in computational chemistry have begun to offer solutions. Computational methods and tools, such as quantum mechanical calculations, molecular modeling, and machine learning algorithms, have become invaluable in predicting reaction pathways and selectivity.
- Quantum Mechanical Calculations allow for the simulation of electronic structures and reaction mechanisms, providing insights into the factors that influence selectivity.
- Molecular Modeling helps visualize potential reaction outcomes, guiding the design of molecules with desired selectivities.
- Machine Learning Algorithms can analyze vast datasets of chemical reactions to predict outcomes based on patterns and trends.
These computational approaches enable chemists to explore potential reaction pathways before physical experimentation, saving time and resources in the laboratory.
Enhancing Selectivity
Strategies and Advancements
Overcoming the challenges of achieving high selectivity in chemical reactions has led to several innovative strategies and advancements:
- Design of Chiral Catalysts: The development of chiral catalysts has been a game-changer in enhancing stereoselectivity, allowing for the preferential formation of one enantiomer over another. These catalysts are particularly crucial in the synthesis of chiral drugs.
- Directed Functionalization: Using directing groups or templates that guide reactants to specific sites on a molecule can significantly improve regioselectivity. This strategy is widely employed in the synthesis of complex organic molecules.
- Reaction Condition Optimization: Fine-tuning reaction conditions such as temperature, solvent, and pressure can have a profound effect on selectivity. High-throughput screening techniques allow for the rapid assessment of multiple conditions to find the optimal set for a desired selectivity.
- Computational Chemistry: As mentioned, computational tools not only predict but also help in designing reactions with enhanced selectivity. By understanding the electronic and steric landscape of reactants, chemists can manipulate conditions to favor certain pathways.
Frequently Asked Questions
What is regioselectivity?
Regioselectivity in chemical reactions refers to the preference of a reaction to occur at one directional site over another within a molecule, leading to the selective formation of one constitutional isomer over others. This concept is crucial in organic synthesis, as it determines the specific location within a molecule where a reaction takes place, thereby influencing the structure and properties of the resulting compound.
How does stereoselectivity affect a chemical reaction?
Stereoselectivity affects a chemical reaction by dictating the spatial arrangement of the atoms or groups in the product molecule. It is concerned with generating products that have specific three-dimensional orientations. This selectivity is paramount in the synthesis of molecules that must exhibit a particular configuration to be biologically active or functionally effective, making it a cornerstone in the development of pharmaceuticals and other application-specific compounds.
Why are regioselectivity and stereoselectivity important in organic synthesis?
Regioselectivity and stereoselectivity are important in organic synthesis because they allow chemists to predict and control the outcomes of chemical reactions, enabling the precise construction of complex molecules. Mastery over these selectivities facilitates the efficient synthesis of compounds with desired physical, chemical, and biological properties. This control is essential for the development of new drugs, materials, and various organic products, making selectivity a key factor in innovative chemical research.
Conclusion
The concepts of regioselectivity and stereoselectivity are foundational pillars in the realm of organic chemistry. They not only enrich our understanding of chemical reactions but also empower chemists to steer these reactions towards desired outcomes. Mastery of these concepts enables the deliberate synthesis of molecules with specific configurations and properties, underscoring the art and science of chemistry in creating compounds that benefit humanity.
As we continue to explore and manipulate the molecular world, the significance of regioselectivity and stereoselectivity will only grow. These principles guide the hands of chemists as they craft the next generation of pharmaceuticals, materials, and chemicals, reflecting the ongoing journey of discovery and innovation in the chemical sciences. Through their application, we are able to harness the full potential of organic synthesis, paving the way for advancements that touch every aspect of our lives.