Electrochemistry plays a pivotal role in the scientific world, bridging the gap between electrical energy and chemical reactions. It’s a field that not only fascinates researchers but also has extensive applications in various industries, including energy, pharmaceuticals, and materials science. Central to understanding electrochemistry are the concepts of reduction potential and reducing power, each a fundamental aspect that predicts how substances interact in oxidation-reduction (redox) reactions.
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. It is expressed in volts (V) and is determined under standard conditions. Conversely, reducing power refers to a substance’s ability to donate electrons, a property that isn’t quantified by a single measure but is inferred from various chemical characteristics. While reduction potential gives a quantitative way to predict the direction of redox reactions, reducing power provides a qualitative insight into the reactivity of a substance.
Both reduction potential and reducing power are crucial for predicting the outcome of chemical reactions, yet they serve different roles. Reduction potential is used to compare the relative strengths of electron acceptors, while reducing power looks at the propensity of a species to act as an electron donor. Understanding these concepts allows chemists to manipulate reactions for desired outcomes, highlighting the importance of these measurements in both academic and practical applications.
Basics of Electrochemistry
Electrochemistry is a fascinating branch of chemistry that deals with the interrelation between electrical energy and chemical reactions. This field of study is not only crucial for understanding how chemical processes can generate electricity but also for exploring how electrical energy can drive chemical changes. The principles of electrochemistry are fundamental to developing batteries, electroplating metals, and electrochemical sensors, among other applications.
Electric Potential
Definition and Relevance
Electric potential, often referred to as electrode potential, is the ability of a system to do work via electric force. It is measured in volts (V) and is a key concept in electrochemistry because it represents the driving force behind the movement of electrons from one atom or molecule to another in redox reactions. This movement is what generates electric current in electrochemical cells.
Electric potential is essential for understanding how batteries work, how metals corrode, and how we can control electroplating processes. It’s the difference in electric potential that prompts electrons to move, leading to various chemical phenomena.
Oxidation and Reduction
Definitions
In the realm of electrochemistry, oxidation and reduction are processes that involve the transfer of electrons between reactants. Oxidation is the loss of electrons from a molecule, atom, or ion, while reduction is the gain of electrons.
Examples
- Oxidation: Iron rusting is a classic example of oxidation, where iron (Fe) loses electrons to form iron oxide (Fe₂O₃) in the presence of oxygen and moisture.
- Reduction: The reduction of copper ions (Cu²⁺) in a solution to copper metal (Cu) on the surface of a metal electrode involves the gain of electrons.
These examples highlight the complementary nature of oxidation and reduction reactions, often abbreviated as redox reactions. In any chemical reaction, the loss of electrons by one substance (oxidation) is always accompanied by the gain of electrons by another (reduction).
Reduction Potential
Concept Overview
Definition
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thus be reduced. It is expressed in volts (V) and is determined under standard conditions, which typically include a concentration of 1 molar, pressure of 1 atmosphere, and a temperature of 25°C (298 K).
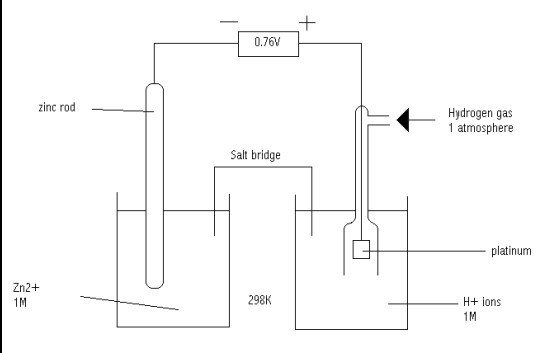
How it is Measured
To measure reduction potential, a standard hydrogen electrode (SHE) is used as a reference. The SHE has a defined potential of 0 volts. By connecting another electrode to the SHE in an electrochemical cell, the difference in electric potential between them can be measured, providing the reduction potential of the electrode in question.
Factors Affecting Reduction Potential
Nature of the Ion
The chemical identity of an ion influences its reduction potential. Atoms with a greater affinity for electrons have higher reduction potentials.
Charge
The charge of an ion affects its ability to gain electrons. Typically, ions with higher positive charges have higher reduction potentials because they are more likely to gain electrons to attain stability.
Solvent Effects
The solvent can also impact the reduction potential. Solvents that stabilize ions or facilitate the movement of electrons can affect the ease with which a chemical species is reduced.
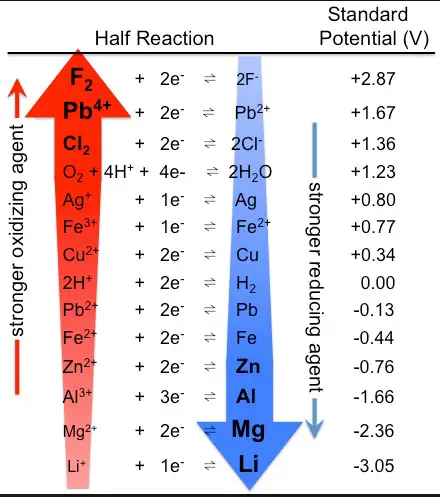
Standard Reduction Potentials
Table and Interpretation
Standard reduction potentials are tabulated values that allow chemists to predict the direction of redox reactions. These values are referenced against the standard hydrogen electrode, which has a potential of 0 volts. By comparing the reduction potentials of two species, one can predict which one is more likely to be reduced (gain electrons) and which one is more likely to be oxidized (lose electrons).
Use in Predicting Reaction Direction
The standard reduction potential helps in determining the spontaneity of redox reactions. If the potential of the reducing agent is higher than that of the oxidizing agent, the reaction is likely to proceed in the forward direction. This principle is essential for designing batteries and other electrochemical cells, where a spontaneous flow of electrons is necessary for the device to function.
Reducing Power
Concept Overview
Reducing power is a term used to describe a substance’s ability to donate electrons in a chemical reaction. This property is crucial in determining how substances interact in oxidation-reduction (redox) reactions. A higher reducing power means a substance is more likely to donate electrons to another substance, effectively reducing it. This concept is fundamental in fields like organic chemistry, metallurgy, and electrochemistry.
Definition
Reducing power refers to the tendency of a compound or element to give up electrons during chemical reactions. This property is opposite to oxidation potential, focusing instead on the capacity for electron donation.
Relation to Electron Donation
The core of reducing power lies in its relationship with electron donation. Substances with high reducing power readily lose electrons, facilitating the reduction of other substances. This electron transfer is the backbone of redox reactions, where electron donors (reducing agents) and acceptors (oxidizing agents) interact.
Factors Influencing Reducing Power
Several key factors affect the reducing power of a substance:
Atomic Size
- Larger atomic size generally correlates with greater reducing power. This is because electrons in larger atoms are further from the nucleus and are therefore easier to donate.
Ionization Energy
- Lower ionization energy means it takes less energy to remove an electron from an atom, enhancing its reducing power. Atoms with low ionization energies are more likely to act as reducing agents.
Electronegativity
- Lower electronegativity is associated with higher reducing power. Elements that are less electronegative are more inclined to give up electrons, making them effective reducing agents.
Measuring Reducing Power
Common Methods and Scales
Reducing power can be assessed through various methods, including:
- Electrochemical techniques, where the ease of electron donation is measured under controlled conditions.
- Chemical reactivity studies, observing how substances react with known oxidizing agents.
Practical Applications
The concept of reducing power is applied in:
- Synthesis of chemicals, where reducing agents are used to produce desired products.
- Battery technology, selecting materials with appropriate reducing power for electrodes.
- Metal extraction, where reducing agents are employed to extract metals from ores.
Comparison
Key Differences
Theoretical Foundation
- Reduction potential is rooted in the tendency to gain electrons, while reducing power focuses on the ability to donate electrons.
Measurement and Units
- Reduction potential is measured in volts (V), providing a quantitative value. In contrast, reducing power is often assessed qualitatively through reactivity patterns.
Relation to Chemical Reactivity
How Each Predicts or Influences Reactivity
- Reduction potential helps predict the direction of redox reactions by comparing potentials of reactants.
- Reducing power indicates the likelihood of a substance acting as a reducing agent, influencing reaction pathways.
Application in Industry
Examples of Practical Applications
- Pharmaceuticals: Reducing agents are used to synthesize complex molecules in drug development.
- Energy Storage: Materials with high reducing power are selected for battery anodes to enhance efficiency and capacity.
- Corrosion Prevention: Substances with appropriate reducing power are chosen for coatings and treatments to protect metals.
FAQs
What is Electrochemistry?
Electrochemistry is the branch of chemistry that deals with the relationship between electrical energy and chemical reactions. It involves the study of chemical changes that produce electricity and the use of electrical energy to bring about chemical transformations.
How is Reduction Potential Measured?
Reduction potential is measured using a standard hydrogen electrode (SHE) as the reference, under standard conditions (25°C, 1M concentration, and 1 atm pressure). The potential difference between the SHE and the electrode in question gives the reduction potential of the substance.
Why is Reducing Power Important?
Reducing power is important because it indicates a substance’s ability to donate electrons in a redox reaction. This property is crucial in many chemical processes, such as corrosion, battery operation, and in the synthesis of various chemicals and pharmaceuticals.
How Do Reduction Potential and Reducing Power Affect Chemical Reactions?
Reduction potential and reducing power directly influence the direction and feasibility of chemical reactions. A higher reduction potential means a greater tendency to gain electrons, while a strong reducing power indicates an eagerness to donate electrons. Together, they help predict whether a redox reaction will occur spontaneously.
Conclusion
In the complex landscape of electrochemistry, reduction potential and reducing power stand out as essential indicators of how substances behave in redox reactions. They are not just theoretical concepts but practical tools that guide chemists in predicting reaction outcomes and in designing new compounds and materials. The nuanced understanding of these properties enriches our ability to harness chemical reactions, advancing both scientific knowledge and technological innovation.
Recognizing the distinct yet interconnected roles of reduction potential and reducing power illuminates the intricate balance of forces driving chemical processes. As we continue to explore the depths of chemical reactivity, these concepts will remain vital in our quest to develop more efficient, sustainable, and innovative solutions across a wide array of scientific and industrial fields.
