Radon and radium are two naturally occurring radioactive elements that, despite their similar names, possess distinct properties and implications for health and safety. Both elements are part of the decay chains of heavier elements such as uranium and thorium, which are found in varying amounts within the Earth’s crust. This connection often leads to confusion regarding their specific characteristics and risks.
Radon is a colorless, odorless gas that emerges from the decay of radium and is considered a significant health risk due to its radioactive nature and ability to accumulate in homes. Radium, on the other hand, is a radioactive metal that emits radiation and can also pose serious health risks if mishandled or ingested. Both elements are critical to understand in terms of environmental science and public health due to their pervasive presence and potential hazards.
Despite their hazardous properties, radon and radium are also valuable in certain industrial and medical applications. Their controlled use underlines the importance of understanding their behaviors and impacts. Knowing how to detect and manage these elements can mitigate health risks and environmental impacts, making the knowledge of their differences essential.
Radon Explained
Definition and Characteristics
Radon is a radioactive gas that is colorless, odorless, and tasteless. It forms naturally from the decay of uranium, which is found in nearly all soils and rocks. As a noble gas, radon is chemically inert and does not chemically react with other elements under normal conditions. Its radioactivity makes it a significant concern for health, particularly because it cannot be detected by human senses.
Sources in the Environment
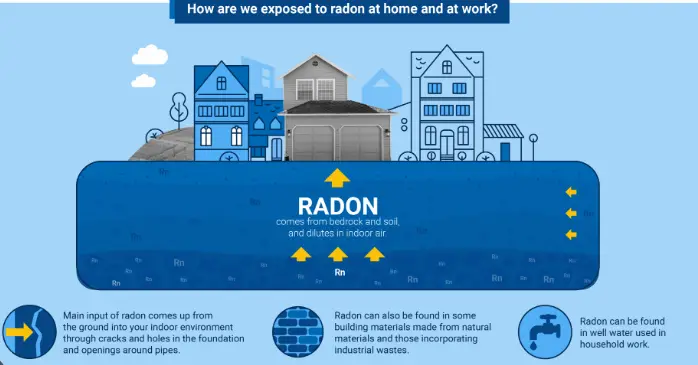
Radon is primarily released from the ground, especially from soil and rocks containing uranium and thorium. It can seep into buildings through cracks in walls, floors, and foundations, accumulating to levels that can pose health risks. Other sources include:
- Building materials such as granite and certain concretes
- Well water that releases radon into the air during usage
Radium Explained
Definition and Characteristics
Radium is a radioactive metal. It shines with a faint blue light and is heavier than most other elements. It is highly radioactive, with its most stable isotope, radium-226, having a half-life of about 1,600 years. Radium decays into radon gas, linking the two in a natural decay chain.
Natural Occurrences
Radium is found in small amounts in ores such as uraninite, often mined for uranium. The distribution of radium is closely tied to that of uranium due to their common origin in the decay process. Natural occurrences include:
- Uranium and thorium ores
- Phosphate rock formations
- Certain shales
- Groundwater containing dissolved radium
Chemical Properties
Atomic Structure of Radon
Radon has an atomic number of 86 and is the heaviest gas in the noble gas family. It has several isotopes, with radon-222 being the most stable and common in the environment. The atomic structure allows radon atoms to be completely surrounded by eight electrons, following the octet rule, which makes them chemically stable.
Atomic Structure of Radium
Radium, with an atomic number of 88, sits two places right of radon on the periodic table. Its electronic configuration allows it to readily lose electrons, making it more chemically reactive than radon. Radium typically forms compounds by replacing calcium due to their similar ionic radii, particularly in bones when ingested.
Comparative Analysis
Comparing radon and radium:
- Radon is a gas at room temperature; radium is a solid.
- Radon is inert; radium is chemically reactive.
- Both are highly radioactive, but radium is typically more hazardous in direct contact due to its solidity and tendency to form compounds.
Physical Properties
State at Room Temperature
Radon exists as a gas at room temperature, making it unique among the other elements in its group. Radium, in contrast, is a solid metal under the same conditions.
Radioactivity Levels
Both radon and radium are highly radioactive. Radon’s ability to move freely as a gas increases the risk of inhalation, while radium’s solid state allows it to be incorporated into materials and possibly ingested.
Health Risks
Health Effects of Radon Exposure
Exposure to radon can lead to lung cancer. The risk increases significantly with higher concentrations and prolonged exposure. Radon is the second leading cause of lung cancer after smoking and poses the highest risk to smokers and former smokers.
Health Effects of Radium Exposure
Radium exposure can cause bone cancer and other disorders, as radium behaves like calcium and can replace it in the bones. Other effects include acute radiation syndrome if ingested or handled improperly.
Comparative Risks
While both elements are dangerous, the risks associated with radon are more widespread due to its gaseous state and the commonality of exposure in homes. Radium, though less frequently encountered, poses severe risks in cases of direct contact or ingestion.
Detection and Measurement
Techniques for Detecting Radon
Detecting radon involves a range of methods, suitable for both short-term and long-term monitoring:
- Charcoal canisters are used for short-term testing and are placed in the lowest livable space of a home for several days before being sent to a lab.
- Alpha track detectors, used for long-term testing, record the alpha particles emitted by radon decay. These detectors are placed in a home for months to provide a more accurate assessment of average radon levels.
- Continuous radon monitors offer real-time measurements and are often used by professionals to assess radon levels before and after mitigation.
Techniques for Detecting Radium
Detecting radium requires different techniques due to its solid state:
- Gamma spectrometry is used to measure radium content in samples by detecting the gamma rays emitted during radioactive decay.
- Liquid scintillation counting is another technique where a sample is mixed with a scintillation cocktail. When radium decays, it emits light that is counted and analyzed.
- Radon emanation techniques measure the radon produced by radium decay as an indirect way of quantifying radium.
Uses and Applications
Industrial Uses of Radon
Despite its risks, radon has several applications:
- Healthcare, specifically in radiotherapy, where it is used to target and kill cancerous cells.
- Geological surveys utilize radon measurements to predict earthquakes and explore for underground resources like oil and uranium.
Industrial Uses of Radium
Radium’s uses include:
- Luminous paints, historically used in watch dials and aircraft switches.
- Medical treatments, particularly in brachytherapy, where small amounts of radium are used to treat cancers by being placed near or within the tumor.
Legal and Safety Regulations
Regulations for Handling Radon
Regulations for radon focus primarily on reducing indoor air levels:
- Building codes in many countries now require radon-resistant construction techniques.
- Health and safety guidelines recommend regular radon testing in homes and workplaces, with mitigation actions if levels exceed EPA-recommended limits.
Regulations for Handling Radium
Handling radium is strictly controlled due to its high radioactivity:
- Storage and disposal of radium must adhere to stringent nuclear safety regulations.
- Use in products has been greatly restricted, especially in consumer goods, to minimize exposure.
Mitigation Strategies
Radon Mitigation in Homes
Effective strategies to reduce radon levels in homes include:
- Sealing cracks in floors and walls to prevent radon entry.
- Installing radon mitigation systems, such as sub-slab depressurization, where a fan and vent pipe system draws radon from beneath the house and expels it outside.
Handling Radium Safely in Industries
Safety protocols for radium include:
- Proper training for workers on handling techniques and potential risks.
- Use of shielding to protect workers from radiation exposure.
- Regular monitoring of both workplace air and worker health.
Environmental Impact
Long-term Effects of Radon on the Environment
Radon contributes to environmental radioactivity, particularly in areas with high natural uranium content. Its effects are more pronounced indoors but can also impact outdoor air quality and ecosystems near radon-emitting sites.
Long-term Effects of Radium on the Environment
Radium’s effects on the environment include:
- Contamination of water bodies from industrial discharge, which can lead to bioaccumulation in aquatic life.
- Soil contamination near radium-rich sites, affecting plant life and potentially entering the food chain.
Frequently Asked Questions
What is radon?
Radon is a radioactive gas that results from the decay of radium in the Earth’s crust. It is invisible, tasteless, and odorless, making it a hidden danger in many homes and buildings where it can accumulate to harmful levels.
How does radium differ from radon?
While radium is a heavy, radioactive metal found in rocks and soil, radon is a gas produced by the decay of radium. Radon is more immediately dangerous in everyday environments due to its gaseous state, which allows it to be inhaled.
What are the health risks of radon?
Long-term exposure to elevated levels of radon can lead to lung cancer. Radon is the second leading cause of lung cancer after smoking and the leading cause among non-smokers.
How can radon be detected?
Radon levels can be detected using various testing kits and devices designed for home or professional use. These tests are crucial for assessing the radon levels in indoor environments, particularly in areas known for high radium content in the ground.
Are there safe levels of radium exposure?
No level of radium exposure is considered completely safe, but certain regulatory limits aim to control its use and manage its risks, especially in drinking water and workplace environments.
Conclusion
The distinctions between radon and radium, though nuanced, are critically important for both health and environmental management. Understanding these differences helps in formulating effective strategies for monitoring, regulation, and mitigation to protect public health. Both elements demonstrate the intricate balance between natural phenomena and human health implications.
Awareness and education about radon and radium are key in managing their presence in our environment. By implementing safety measures and regular monitoring, communities can significantly reduce the risks associated with these radioactive elements, leading to safer living and working conditions.