Pyrrolidine and piperidine are two pivotal nitrogen-containing heterocyclic organic compounds that play crucial roles in the field of organic chemistry. While both compounds share some similarities in their ring structures, their applications and properties differ significantly, influencing various scientific and industrial processes. The nuanced differences between these compounds underscore their unique chemical characteristics and their impact on synthesis and product development.
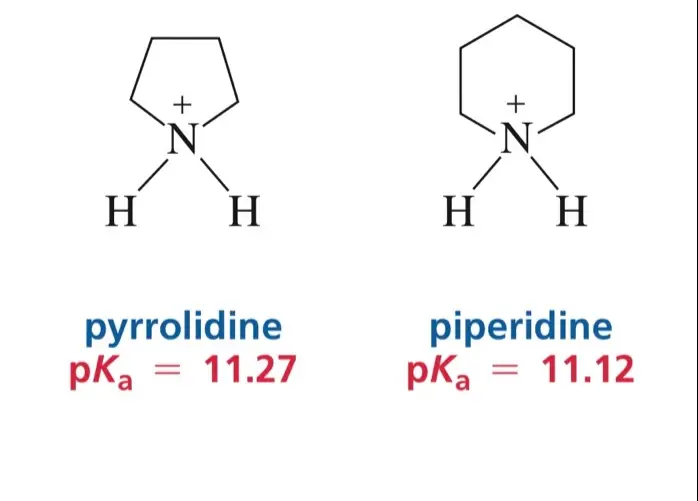
Pyrrolidine is a five-membered ring with four carbon atoms and one nitrogen atom, whereas piperidine, also a five-membered ring, contains five carbon atoms and one nitrogen atom. These structural distinctions affect their physical properties, reactivity, and the range of applications in pharmaceuticals and industrial chemistry. Understanding these differences is key for chemists and researchers working in drug development and synthetic organic chemistry.
The study of pyrrolidine and piperidine extends beyond their basic chemical structures, touching on aspects such as synthesis methods, physical properties, and their roles in biological systems. These compounds are foundational in creating a multitude of chemical reactions and are integral in the design of new drugs and advanced materials. Their continued relevance in scientific research highlights the dynamic nature of chemistry and its ability to address complex biological and industrial challenges.
Chemical Structure
Pyrrolidine Structure
Pyrrolidine is a cyclic secondary amine, consisting of a five-membered ring containing four carbon atoms and one nitrogen atom. The molecular formula for pyrrolidine is C4H9N. This structure is saturated, meaning it does not contain double bonds, which contributes to its stability under normal conditions. The hydrogen atoms attached to the carbon in the ring further define its chemical behavior and reactivity.
Piperidine Structure
Piperidine, similarly to pyrrolidine, is a five-membered ring. However, its structure includes five carbon atoms along with a single nitrogen atom, with the molecular formula being C5H11N. Unlike pyrrolidine, piperidine’s additional carbon atom influences its physical and chemical properties, such as boiling points and solubility, making it slightly less volatile.
Comparative Analysis
Comparing pyrrolidine and piperidine, the key difference lies in the ring’s carbon count, which affects various chemical and physical properties. Both structures are pivotal in organic chemistry, especially in synthesizing complex molecules. This comparative analysis allows chemists to select the appropriate compound based on the required reactivity and desired synthesis outcome.
Physical Properties
Boiling and Melting Points
- Pyrrolidine has a boiling point of about 87°C and a melting point of -63°C.
- Piperidine boils at around 106°C and melts at -7°C.
These boiling and melting points reflect how each compound interacts with heat, which is crucial for practical applications, especially in synthesis and production environments.
Solubility and Density
- Pyrrolidine is highly soluble in water and most organic solvents due to its polar nature. Its density is about 0.866 g/cm³ at 25°C.
- Piperidine also shows good solubility in water and organic solvents, with a density of approximately 0.862 g/cm³ at 25°C.
The solubility and density of these compounds are important for chemists to understand as they influence how these chemicals can be handled, stored, and used in industrial processes.
Synthesis Methods
Pyrrolidine Synthesis
Pyrrolidine can be synthesized through several methods, including the reduction of pyrrolidone. The steps typically involve:
- Starting with the lactam (cyclic amide) of pyrrolidine known as pyrrolidone.
- Reducing pyrrolidone using a strong reducing agent like lithium aluminum hydride.
This synthesis route is favored for its efficiency and the high purity of pyrrolidine that it yields.
Piperidine Synthesis
Piperidine is commonly synthesized by hydrogenating pyridine, a process involving:
- The addition of hydrogen to pyridine in the presence of a metal catalyst at high pressure and temperature.
- This method transforms the aromatic ring of pyridine into a saturated piperidine ring.
Key Differences in Synthesis
The synthesis of pyrrolidine and piperidine highlights distinct approaches due to their structural differences. Pyrrolidine synthesis often involves ring expansion or reduction reactions, whereas piperidine typically requires hydrogenation of an aromatic ring. These methods reflect the compounds’ unique reactivities and are chosen based on the desired scale of production and application specificity.
Applications
Pharmaceutical Uses
Both pyrrolidine and piperidine are integral in the pharmaceutical industry. They are used to create a variety of therapeutic compounds. For instance:
- Pyrrolidine derivatives are key in developing antiviral drugs, including treatments for HIV.
- Piperidine is used in producing analgesics and antihistamines, enhancing many medicinal formulations.
Industrial Applications
In the industrial realm, these compounds have versatile uses. Pyrrolidine and piperidine are used in:
- Solvent production.
- Catalysts in chemical reactions.
- Creating polymers and plastics.
Reactivity and Stability
Chemical Reactivity
Pyrrolidine and piperidine exhibit distinct reactivity profiles due to their structural differences. Pyrrolidine tends to participate in nucleophilic addition reactions because of the electron-donating effect of the nitrogen in the ring. This makes it reactive towards electrophiles, such as alkyl halides, enabling the formation of various organic compounds.
Piperidine, on the other hand, also shows significant reactivity towards electrophiles but can stabilize positive charges better due to its additional carbon atom. This characteristic is particularly useful in synthetic organic chemistry, where piperidine is used to open epoxides or as a base in deprotonation reactions.
Stability Under Various Conditions
Both compounds are stable under normal atmospheric conditions but behave differently under increased temperatures or in the presence of strong acids or bases.
- Pyrrolidine is relatively stable but can be oxidized to the corresponding lactam, pyrrolidone, under oxidative conditions.
- Piperidine can withstand higher temperatures better than pyrrolidine but is susceptible to oxidation, forming N-oxidized products which are useful intermediates in organic synthesis.
Biological Importance
Role in Biochemistry
Pyrrolidine and piperidine rings are present in many bioactive molecules, playing critical roles in the structure and function of various biological compounds. For example, proline, an amino acid containing the pyrrolidine ring, is crucial in protein folding and structure. Piperidine structures are found in several alkaloids and neurotransmitters, influencing biological pathways and cellular communication.
Therapeutic Effects
Both pyrrolidine and piperidine derivatives are utilized in the development of a range of therapeutic agents.
- Pyrrolidine derivatives are known for their effectiveness in antiviral therapies, particularly in medications targeting HIV and hepatitis C.
- Piperidine derivatives are widely used in pain management and allergy treatments, as well as in medications for neurological disorders such as Parkinson’s disease and schizophrenia.
Environmental Impact
Safety and Handling
Safety in handling pyrrolidine and piperidine is paramount due to their volatile nature and potential health hazards.
- Pyrrolidine can cause skin and respiratory irritation and must be handled with adequate protective gear in a well-ventilated area.
- Piperidine poses similar risks and additionally can emit harmful fumes under high heat, necessitating stringent controls during its use in industrial applications.
Disposal Considerations
Proper disposal of pyrrolidine and piperidine is crucial to minimize their environmental impact. Guidelines typically recommend:
- Neutralization of these compounds before disposal to prevent release into the environment.
- Following local regulations that may dictate specific disposal methods such as incineration or treatment with activated carbon to capture and break down the chemicals safely.
FAQs
What is Pyrrolidine?
Pyrrolidine is an organic compound with the molecular formula C4H9N. It forms a five-membered ring structure predominantly used in the synthesis of various pharmaceuticals. Its presence is crucial in many natural bioactive compounds, enhancing their pharmacological properties.
How is Piperidine Used in Industry?
Piperidine is extensively used in the chemical industry, particularly in the synthesis of pharmaceuticals, as a catalyst, and in the production of polymers. Its reactivity makes it a valuable building block in organic synthesis.
Can Pyrrolidine and Piperidine Interchange in Synthesis?
While both pyrrolidine and piperidine are used in organic synthesis, they generally cannot be interchanged due to their distinct reactivity and resulting chemical properties. Each plays unique roles depending on the specific chemical environment and desired reaction outcomes.
What are the Safety Concerns with Piperidine?
Piperidine is a strong base and can be hazardous if not handled properly. It requires careful management to prevent skin, eye, and respiratory system irritation. Safety data sheets recommend using protective gear and proper ventilation when working with piperidine.
How do Pyrrolidine and Piperidine Differ Chemically?
Chemically, pyrrolidine and piperidine differ primarily in their hydrogen atom placement and the resulting electronic effects in their molecular structures. These differences significantly impact their chemical reactivity and physical properties.
Conclusion
The intricate distinctions between pyrrolidine and piperidine underscore the complexity of organic compounds and their profound impact on modern science and industry. Their unique properties facilitate the development of new chemical reactions and applications, from pharmaceuticals to advanced polymers. Recognizing these differences not only enhances our understanding of chemical fundamentals but also propels the innovation of new technologies and solutions in various fields.
As research continues to explore the potential of these compounds, the future holds promising advancements that could lead to groundbreaking applications. The ongoing study of their properties and applications is essential for the continued evolution of scientific practices and industrial applications, highlighting the ever-growing relationship between organic chemistry and technological progress.