Organic chemistry, a branch of chemistry concerned with the structure, properties, and reactions of organic compounds and materials, introduces us to a myriad of complex molecules that play crucial roles in various scientific and industrial domains. Among these, heterocyclic compounds stand out for their unique structures and functionalities. Two notable members of this group are pyridine and pyrimidine, both of which feature prominently in the synthesis of numerous pharmaceuticals, agrochemicals, and dyes.
Pyridine and pyrimidine are aromatic heterocyclic compounds characterized by their ring structures containing nitrogen atoms. Pyridine is a six-membered ring compound with one nitrogen atom, while pyrimidine also is a six-membered ring but with two nitrogen atoms at positions 1 and 3. This structural difference underlies the distinct chemical behaviors, applications, and significance of each compound in various fields, including medicine, agriculture, and environmental science.
Despite their structural similarities, pyridine and pyrimidine exhibit different physical and chemical properties due to the variance in the number and position of nitrogen atoms within their rings. These differences extend to their reactivity, boiling and melting points, solubility in water, and the roles they play in the synthesis of other compounds. Understanding these properties is not only crucial for chemists but also for those involved in the development of new pharmaceuticals, agrochemicals, and materials with specific characteristics.
Basic Definitions
Pyridine
Chemical Structure and Characteristics
Pyridine is a basic heterocyclic organic compound. Its structure consists of a six-membered ring with five carbon atoms and one nitrogen atom. The presence of the nitrogen atom contributes to pyridine’s basicity and distinguishes its chemical behavior from benzene, despite their structural similarity. Pyridine is a colorless liquid with a distinctive, somewhat unpleasant odor, often compared to fish.
- Solubility: Pyridine is miscible with water and most organic solvents, a characteristic that enhances its utility in various chemical reactions and industrial applications.
- Boiling Point: With a boiling point of 115°C, pyridine is relatively volatile.
- Electron Distribution: The nitrogen atom in pyridine contributes to electron density around the ring, influencing its reactivity.
Pyrimidine
Chemical Structure and Characteristics
Pyrimidine is another essential heterocyclic compound, composed of a six-membered ring with two nitrogen atoms at positions 1 and 3. This structure forms the backbone of several important biological molecules, including thymine, cytosine, and uracil, which are vital components of DNA and RNA.
- Solubility: Pyrimidine is less soluble in water than pyridine, but still shows good solubility in organic solvents.
- Boiling Point: It has a higher boiling point compared to pyridine, reflecting the increased stability provided by the additional nitrogen atom.
- Electron Distribution: The presence of two nitrogen atoms affects the electron distribution across the ring, making pyrimidine less basic than pyridine.
Historical Context
Discovery of Pyridine
Origin and Initial Uses
Pyridine was first isolated in the 19th century from coal tar, a thick black liquid produced during coal gasification. Early on, its unique properties spurred interest for use in various industrial processes.
- Initially, pyridine found use as a solvent and as a reagent in organic synthesis.
- Its ability to dissolve a wide range of compounds made it valuable in the manufacture of dyes, rubber, and medicines.
Discovery of Pyrimidine
Origin and Initial Uses
The discovery of pyrimidine dates back to the late 19th century. Unlike pyridine, pyrimidine and its derivatives were identified through studies of nucleic acids, where they play a critical role in the structure of DNA and RNA.
- Pyrimidine derivatives, notably thymine, cytosine, and uracil, were recognized for their fundamental role in genetic coding and protein synthesis.
- This discovery paved the way for significant advancements in biochemistry and molecular biology.
Chemical Properties
Structure and Bonding
Comparison of Ring Structure
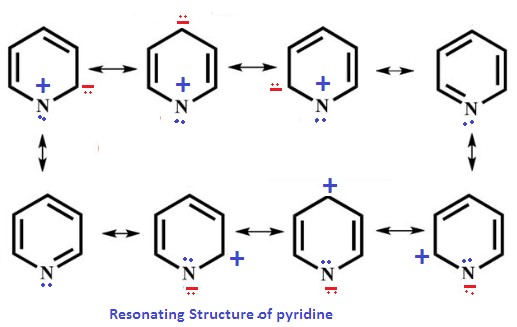
The ring structures of pyridine and pyrimidine are central to their chemical behavior. Pyridine’s single nitrogen atom contributes to its aromaticity, similar to benzene but with distinct reactivity due to the nitrogen. Pyrimidine’s structure, with two nitrogen atoms, results in a ring that is less aromatic but more stable and less reactive towards electrophilic substitution reactions.
Type of Bonding and Electron Distribution
- Pyridine: The nitrogen atom in pyridine donates electron density to the ring, making it electron-rich. This affects its bonding with other atoms and molecules, facilitating the formation of complexes with metals and participation in nucleophilic reactions.
- Pyrimidine: The two nitrogen atoms in pyrimidine withdraw electron density from the carbon atoms, making the ring electron-deficient compared to pyridine. This influences its chemical stability and reactivity.
Physical Properties
Boiling Points, Melting Points, and Solubility
The physical properties of pyridine and pyrimidine reflect their structural differences:
- Pyridine has a lower melting point and a boiling point of 115°C, indicating its relatively volatile nature. Its miscibility with water and organic solvents underlines its role as a versatile solvent.
- Pyrimidine, with its higher boiling point, shows greater thermal stability. Its solubility characteristics are affected by the presence of two nitrogen atoms, making it less soluble in water than pyridine but still soluble in many organic solvents.
Chemical Behavior
Reactivity with Other Compounds
Pyridine and pyrimidine exhibit distinct reactivity patterns due to their differing electronic configurations:
Biological Importance
Role in Nature
Both pyridine and pyrimidine are more than just laboratory chemicals; they’re integral to life itself. Pyrimidine, in particular, forms the backbone of nucleic acids—DNA and RNA—where its derivatives cytosine, thymine, and uracil are among the basic building blocks of genetic information. Pyridine, though less prevalent naturally, plays a vital role in the structure of vitamins such as niacin and pyridoxine, which are essential for metabolic processes.
Natural Occurrence and Biological Roles
- Pyrimidine bases are crucial for the storage and transmission of genetic information in organisms, from the simplest bacteria to humans.
- Pyridine nucleotides are involved in energy production and biochemical reactions within cells, highlighting their importance in metabolism.
Applications in Medicine
Use in Pharmaceuticals and Drug Design
The structural characteristics of pyridine and pyrimidine make them valuable scaffolds in drug design and development. Many drugs on the market today contain pyridine or pyrimidine rings, utilized for their pharmacological properties.
- Antibiotics, antiviral drugs, and anticancer agents often incorporate these structures to interact effectively with biological targets.
- The versatility of pyridine and pyrimidine allows for the synthesis of compounds with a wide range of therapeutic effects.
Industrial Uses
Pyridine
Applications in Chemical Synthesis
Pyridine is a key reagent and solvent in the synthesis of various organic compounds. Its basic nature and solvent properties make it indispensable in numerous chemical reactions.
- Used in the synthesis of agrochemicals, pharmaceuticals, and dyes.
- Acts as a catalyst and solvent in condensation reactions.
Role in the Manufacture of Pesticides
Pyridine derivatives play a significant role in the production of pesticides. Their effectiveness in disrupting the life cycle of pests without harming crops makes them valuable in agriculture.
- Herbicides and insecticides containing pyridine are among the most effective means of pest control.
Pyrimidine
Use in Agrochemicals
Similar to pyridine, pyrimidine derivatives are widely used in creating agrochemicals. Their ability to target specific pests and diseases while being safe for crops is noteworthy.
- Pyrimidine-based fungicides protect plants from fungal infections, ensuring crop safety and food security.
Importance in Dyes and Pigments
The structural versatility of pyrimidine makes it a key component in the production of dyes and pigments. These compounds are used in textiles, inks, and various industrial applications, showcasing their broad utility.
- Pyrimidine derivatives provide stability and vibrancy to dyes, enhancing their application range.
Environmental Impact
Biodegradability
The environmental persistence of chemical compounds is a growing concern. Fortunately, both pyridine and pyrimidine are considered biodegradable, albeit at different rates. Microorganisms in soil and water can break down these compounds, minimizing their long-term impact on the environment.
Comparison of Environmental Persistence
- Pyrimidine tends to degrade more slowly than pyridine due to its more complex structure. However, both compounds are eventually broken down by natural processes.
Toxicity
Effects on Human Health and Ecosystems
While pyridine and pyrimidine are invaluable in various industries, their toxicity levels are a concern. Pyridine is known for its unpleasant smell and can be irritating to skin and mucous membranes, but it’s generally not highly toxic. Pyrimidine and its derivatives, being part of biological systems, are usually less harmful but can still pose risks in high concentrations.
- It’s essential to handle these compounds with care, considering their potential environmental and health impacts.
Analytical Methods
Detection and Quantification
Accurate detection and quantification of pyridine and pyrimidine are crucial in environmental monitoring, pharmaceutical manufacturing, and research. Advanced analytical techniques are employed to achieve precise measurements.
- Chromatography and mass spectrometry are among the primary methods used for identifying and quantifying these compounds in various matrices.
Spectral Properties
UV/Vis, IR, NMR Characteristics
The spectral properties of pyridine and pyrimidine are distinctive and can be used for identification and structural analysis.
- UV/Vis spectroscopy provides insights into the electronic transitions in these molecules, useful in determining their concentration and purity.
- Infrared (IR) spectroscopy is employed to study the functional groups and bonding patterns, while nuclear magnetic resonance (NMR) spectroscopy offers detailed information about their molecular structure.
Frequently Asked Questions
What is the main difference between Pyridine and Pyrimidine?
The main difference between pyridine and pyrimidine lies in their ring structure and the number of nitrogen atoms. Pyridine contains a six-membered ring with one nitrogen atom, whereas pyrimidine has a six-membered ring with two nitrogen atoms. This structural difference significantly impacts their chemical properties and applications.
Why are Pyridine and Pyrimidine important in pharmaceuticals?
Pyridine and pyrimidine are foundational structures for many pharmaceutical compounds due to their ability to easily interact with biological systems. Their heterocyclic nature allows for the creation of molecules that can effectively bind to enzymes and receptors, leading to the development of a wide range of drugs for treating diseases such as cancer, viral infections, and cardiovascular conditions.
How do Pyridine and Pyrimidine behave in the environment?
Pyridine and pyrimidine behave differently in the environment due to their distinct chemical structures. Pyridine, being more volatile, tends to evaporate and degrade faster, while pyrimidine, with its additional nitrogen atom, might persist longer and is less volatile. However, both compounds are considered biodegradable to varying extents, eventually breaking down into less harmful substances.
Conclusion
Pyridine and pyrimidine, though closely related, present a fascinating study of how slight differences in molecular structure can lead to varied chemical properties and uses. Their distinct characteristics make them indispensable in the realms of pharmaceuticals, agrochemicals, and industrial chemistry, highlighting the importance of understanding these compounds in the advancement of science and technology.
Their role extends beyond mere chemical interest; pyridine and pyrimidine embody the essence of innovation in drug design and the development of new materials. As researchers continue to explore the potential of these heterocyclic compounds, their significance in contributing to health, agriculture, and environmental safety remains undisputed, underscoring the marvels of chemistry in solving real-world challenges.