Coal remains a cornerstone of the global energy supply, despite the growing emphasis on renewable resources. The quality of coal is paramount, influencing not only its efficiency as a fuel but also its environmental impact. This makes the analysis of coal an essential practice in industries reliant on this fossil fuel.
Proximate and ultimate analyses are two fundamental approaches to understanding coal’s characteristics. Proximate analysis determines coal’s moisture, volatile matter, ash content, and fixed carbon. Conversely, ultimate analysis provides a detailed chemical composition, revealing the percentages of carbon, hydrogen, sulfur, nitrogen, and oxygen.
Given its extensive use in power generation and manufacturing, accurate coal analysis ensures compliance with environmental standards and optimizes its energy output. This analysis not only helps in assessing coal’s combustion characteristics but also in its pricing, purchasing decisions, and suitability for specific industrial applications.
Coal Analysis Basics
What is Coal?
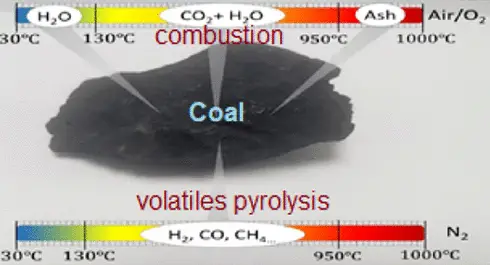
Coal is a black or brownish-black sedimentary rock that is primarily composed of carbon along with varying amounts of other elements, chiefly hydrogen, sulfur, oxygen, and nitrogen. It is formed from the remains of vegetation which has been consolidated between other rock strata and altered by the combined effects of pressure and heat over millions of years. Coal is extracted from the earth through mining and is used as a fuel.
Reasons for Analyzing Coal
Analyzing coal is crucial for several reasons:
- Energy Production: Coal’s role in energy production is well-known. Analyzing its properties helps in assessing how much energy it can produce.
- Environmental Safety: Knowing the chemical makeup of coal can predict the pollutants it may emit when burned.
- Economic Decisions: Analysis aids in determining coal quality, impacting pricing and marketability.
- Regulatory Compliance: Ensures that coal meets local and international environmental standards.
Proximate Analysis Explained
Proximate analysis assesses coal based on its main constituents — moisture, volatile matter, ash content, and fixed carbon. These metrics give a quick and inexpensive assessment of a coal’s quality and suitability for various applications.
Moisture Content
Moisture content is the amount of water found in coal. It affects how coal burns and its overall heat value. High moisture content in coal can lower the temperature at which it burns, reducing its efficiency as a fuel.
Volatile Matter
Volatile matter consists of chemicals in coal that are released as gas or vapor at high temperatures, excluding moisture. This aspect is crucial because it influences the combustion process. Coals with high volatile matter content ignite easily and are highly reactive.
Ash Content
Ash content is the residue left after coal combustion. It is a measure of the non-combustible materials in coal. High ash content can lead to problems in thermal power generation, such as slagging and fouling of equipment.
Fixed Carbon
Fixed carbon refers to the carbon left in coal after all the volatile materials are driven off. This component gives a rough estimate of the heating value of coal. Higher fixed carbon indicates a higher calorific value, making the coal more efficient for power generation.
Ultimate Analysis Defined
Ultimate analysis provides a comprehensive composition of coal by determining its elemental constituents, which include carbon, hydrogen, nitrogen, sulfur, and oxygen. This analysis is essential for understanding the chemical properties that influence its energy value and environmental impact.
Elemental Composition
Carbon
Carbon is the main element in coal, and its percentage directly impacts how much heat coal can generate. Higher carbon content leads to higher energy output.
Hydrogen
Hydrogen in coal contributes to the generation of heat and, combined with oxygen, forms water as a combustion product. The hydrogen content can also indicate the amount of volatile matter coal contains.
Nitrogen
Nitrogen does not contribute to the heating value but is important for understanding the potential emissions of nitrogen oxides, which are regulated due to their impact on air quality.
Sulfur
Sulfur in coal is a critical component in determining the environmental impact of its combustion. Sulfur dioxide, formed when sulfur burns, is a major air pollutant and needs to be managed to meet environmental regulations.
Oxygen
Oxygen is present in coal mostly in organic compounds. It affects the coal’s reactivity and heating value. Lower oxygen content typically indicates a higher calorific value.
Methodologies
Proximate Analysis Methods
Proximate analysis of coal involves several key procedures to assess its quality and composition. The standard methods include:
- Moisture Testing: This involves heating a known weight of coal at a specified temperature until it reaches a constant weight.
- Volatile Matter Testing: Coal is heated in an oxygen-free environment, and the released gases are measured to determine the volatile matter content.
- Ash Testing: Coal is burned completely at a high temperature to determine the amount of non-combustible residue (ash) it contains.
- Fixed Carbon Calculation: This is typically computed by subtracting the sum of moisture, volatile matter, and ash from 100%.
Ultimate Analysis Techniques
Ultimate analysis is more detailed and complex, focusing on the chemical composition of coal. It typically involves:
- Carbon and Hydrogen: Combustion methods where coal is burned in excess oxygen to measure the CO2 and H2O produced.
- Sulfur Analysis: Various methods such as Eschka method, bomb calorimeters, or infrared spectroscopy are used to quantify sulfur content.
- Nitrogen and Oxygen: These elements are often analyzed using advanced methods like Kjeldahl for nitrogen and by difference for oxygen.
Comparative Analysis
Comparative analysis between proximate and ultimate analysis helps stakeholders understand the strengths and limitations of each method in specific applications.
- Proximate Analysis: Offers a quick, economical assessment ideal for initial screening.
- Ultimate Analysis: Provides detailed information on chemical composition critical for precise energy calculations and environmental compliance.
Data Interpretation
Interpreting data from coal analyses involves understanding the implications of each element:
- Energy Value: Correlated directly with fixed carbon and volatile matter.
- Emission Predictions: Sulfur and nitrogen content help predict potential pollution levels.
- Usage Recommendations: Based on moisture and ash content, recommendations can be made for handling and burning coal efficiently.
Practical Applications
The insights gained from coal analysis have practical applications in various industries:
- Power Generation: Adjusting furnace operations based on coal quality to optimize combustion and minimize emissions.
- Cement Manufacturing: Using ash content data to adjust the mix for cement production.
- Steel Industry: Selecting coal grades that reduce impurities and enhance the quality of steel.
Industry Implications
Energy Production
Coal analysis directly impacts energy production by:
- Efficiency Optimization: Knowing the combustion characteristics allows for adjustments in power plant operations to maximize efficiency.
- Fuel Selection: Helps in selecting the right type of coal for different thermal plants based on their technology and emission control systems.
Environmental Impact
The environmental implications of coal use are significant, and analysis helps in mitigating these effects:
- Emissions Control: By understanding the sulfur and nitrogen contents, plants can implement appropriate scrubbing and treatment technologies to reduce emissions.
- Waste Management: Ash content analysis assists in managing the disposal and utilization of coal combustion by-products, potentially in construction materials.
Frequently Asked Questions
What is proximate analysis?
Proximate analysis of coal measures its moisture, volatile matter, ash content, and fixed carbon. These components help in assessing the coal’s quality and performance during combustion, crucial for industrial applications where energy efficiency and output are prioritized.
Why perform ultimate analysis?
Ultimate analysis provides detailed information about the elemental composition of coal, including carbon, hydrogen, sulfur, nitrogen, and oxygen. This data is vital for understanding the burning characteristics of coal, its environmental impact, particularly sulfur-induced air pollution, and energy value.
How do the analyses impact coal use?
The results from proximate and ultimate analyses directly influence how industries use coal. By determining the quality and combustion characteristics of coal, companies can optimize its use in power generation, thus enhancing efficiency and reducing environmental impacts.
Can these analyses predict environmental impact?
Yes, both analyses can help predict the environmental impact of coal usage. Proximate analysis indicates the amount of ash and volatile substances, while ultimate analysis shows the elemental pollutants like sulfur and nitrogen, which are crucial for assessing potential emissions.
Conclusion
Analyzing coal through proximate and ultimate methods offers crucial insights into its composition and combustibility, which in turn influences both its market value and environmental footprint. These analyses ensure that industries can make informed decisions, enhancing efficiency while adhering to environmental regulations.
In light of evolving energy demands and environmental awareness, the role of coal analysis grows increasingly vital. It not only underscores the ongoing need for fossil fuels in today’s energy landscape but also highlights the importance of optimizing their use to minimize environmental impact.

