Protein kinases play a pivotal role in cellular communication and function, acting as key regulators of cell activity. These enzymes facilitate the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process, known as phosphorylation, is crucial for activating or deactivating enzymes and receptor molecules, thus influencing cellular pathways.
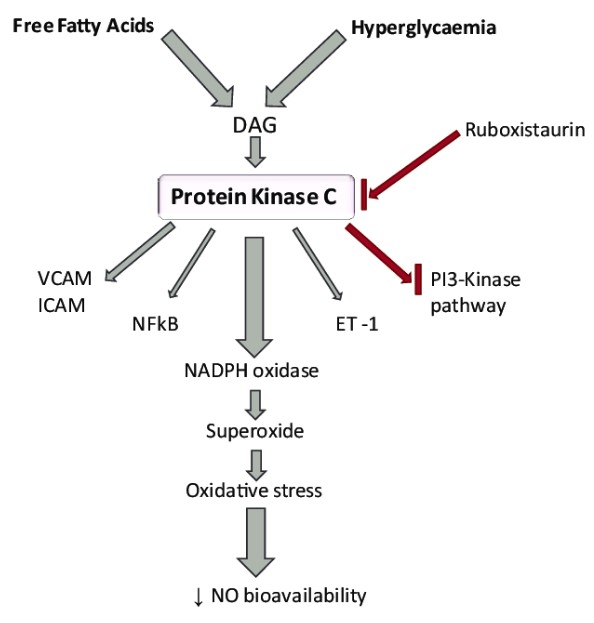
The primary difference between Protein Kinase A (PKA) and Protein Kinase C (PKC) lies in their activation mechanisms and roles within the cell. PKA is activated by cyclic AMP (cAMP), a signaling molecule that responds to various hormonal and other extracellular signals. On the other hand, PKC activation is dependent on the presence of diacylglycerol (DAG) and calcium ions, playing a crucial role in cell signaling pathways that respond to a variety of external stimuli.
Both PKA and PKC are integral to the regulation of numerous cellular processes, including metabolism, gene expression, and cell cycle progression. Their activity is precisely controlled by specific regulatory proteins and mechanisms that ensure cellular functions are carried out correctly. The distinct pathways and mechanisms of action for PKA and PKC underscore the complexity of cellular regulation and the specificity with which cells respond to internal and external cues.
Protein Kinase Basics
Protein kinases play a crucial role in the regulation of cellular activities through the process of phosphorylation, where a phosphate group is added to a protein, thereby altering its function. This action is fundamental to a variety of cellular processes, including signal transduction pathways that help cells respond to their environment.
Role in Cells
Enzymatic Functions
Protein kinases act as enzymes that modify other proteins by chemically adding phosphate groups to them, a process known as phosphorylation. This modification can change the protein’s activity, its interaction with other proteins, and even its location within the cell. Essentially, kinases turn proteins on or off, acting as “switches” for cellular functions such as division, growth, and death.
Signal Transduction Pathways
Signal transduction pathways are critical for cells to communicate with their environment and with each other. Protein kinases are at the heart of these pathways, acting as relays that pass on signals from the cell surface to the interior. Through phosphorylation, they propagate cellular signals that lead to a specific cellular response, such as gene expression, cell growth, or apoptosis.
Types of Kinases
Brief Overview
There are hundreds of kinases in the human body, and they can be broadly categorized based on their substrate specificity—serine/threonine kinases, tyrosine kinases, and dual-specificity kinases. Each type targets specific amino acids in proteins for phosphorylation.
Classification by Target
- Serine/threonine kinases target the amino acids serine and threonine.
- Tyrosine kinases target the amino acid tyrosine.
- Dual-specificity kinases can phosphorylate both serine/threonine and tyrosine residues.
Protein Kinase A (PKA)
Structure
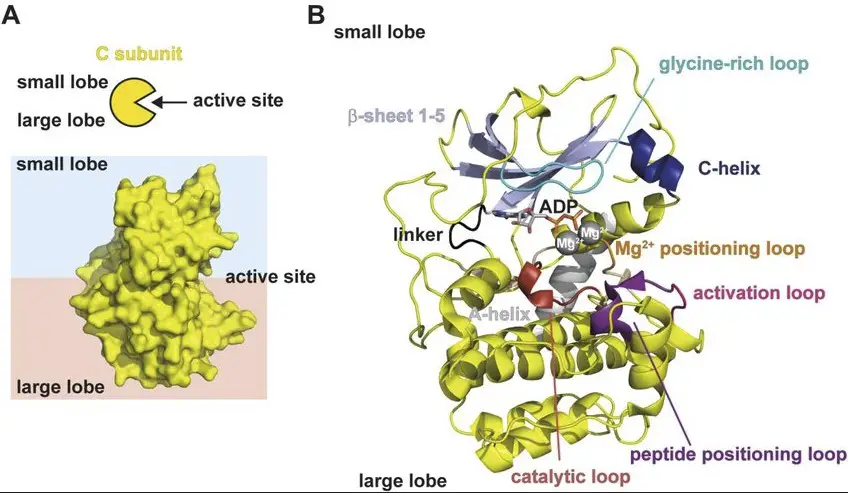
Protein Kinase A, also known as PKA, consists of two main subunits: regulatory (R) and catalytic (C). The regulatory subunits bind cyclic AMP (cAMP), a signaling molecule, and keep the catalytic subunits in an inactive state until cAMP levels rise.
Regulatory and Catalytic Subunits
- Regulatory subunits bind cAMP and inhibit the catalytic subunits.
- Catalytic subunits phosphorylate target proteins when activated.
Activation Mechanism
The activation of PKA is a classic example of how a cellular signal can lead to a cascade of events within the cell. When cAMP levels increase in response to external signals, it binds to the regulatory subunits of PKA, causing a conformational change that releases the catalytic subunits. These freed catalytic subunits can then phosphorylate target proteins, leading to changes in cell behavior.
Function
Role in Cellular Signaling
PKA plays a vital role in cellular signaling, mediating responses to hormones like adrenaline and glucagon. It influences various cellular processes, including metabolism, gene expression, and memory formation.
Examples of PKA Pathways
- Glycogen metabolism: PKA activates enzymes involved in the breakdown of glycogen, a stored form of glucose, thus increasing blood sugar levels.
- Heart rate regulation: PKA phosphorylates proteins in heart cells, increasing heart rate in response to adrenaline.
Regulation
Cyclic AMP (cAMP) Dependency
PKA activity is directly dependent on the levels of cyclic AMP (cAMP) within the cell. cAMP serves as a “second messenger”, relaying signals from first messengers like hormones to PKA and other intracellular targets.
Regulatory Proteins and Mechanisms
The activity of PKA is also regulated by specific proteins that modulate the interaction between cAMP and the regulatory subunits, as well as the localization and stability of the catalytic subunits. This ensures that PKA is activated only under appropriate conditions.
Protein Kinase C (PKC)
Structure
Protein Kinase C (PKC) is a family of kinases with multiple isoforms, reflecting a high degree of diversity in structure and function. Unlike PKA, PKC isoforms are activated by diacylglycerol (DAG) and, in some cases, calcium ions.
Isoforms and Diversity
PKC isoforms are divided into three main groups based on their activation requirements and structure:
- Conventional PKCs (cPKCs) require DAG and calcium for activation.
- Novel PKCs (nPKCs) are activated by DAG but not calcium.
- Atypical PKCs (aPKCs) do not require DAG or calcium for activation.
PKA vs. PKC
Exploring the distinctions between Protein Kinase A (PKA) and Protein Kinase C (PKC) unveils the intricacies of cellular signaling mechanisms. These differences are pivotal in understanding how cells react to various stimuli and maintain homeostasis.
Activation Mechanisms
cAMP vs. Calcium and DAG
PKA activation is fundamentally tied to the levels of cyclic AMP (cAMP), a secondary messenger that facilitates the activation of PKA by binding to its regulatory subunits. This process liberates the catalytic subunits, enabling them to phosphorylate target proteins.
In contrast, PKC activation involves diacylglycerol (DAG) and, in some isoforms, calcium ions. DAG serves as a secondary messenger that, together with calcium in some cases, activates PKC by altering its conformation and localizing it to the cell membrane where it can interact with its substrates.
Cellular Roles
Comparison of Signaling Pathways
The cellular roles of PKA and PKC are both broad and varied, impacting numerous physiological processes. PKA primarily mediates responses to hormones and neurotransmitters, significantly influencing metabolic pathways, gene expression, and memory formation. For instance, PKA regulates glycogen, sugar, and lipid metabolism.
PKC, on the other hand, is more closely associated with the regulation of cell growth, differentiation, and apoptosis. It plays a critical role in the immune response and in the modulation of ion channels and receptors. The diversity of PKC isoforms allows for a wide range of functions, tailored to the specific needs of different cell types and stimuli.
Regulatory Differences
Intracellular Locations
The intracellular locations of PKA and PKC are crucial for their activation and function. PKA is typically found in the cytosol but can be recruited to specific locations within the cell, such as the nucleus, upon activation. This localization is key to targeting specific substrates and achieving precise cellular responses.
PKC isoforms have diverse localizations within the cell, influenced by their activation mechanisms. For example, conventional PKCs are recruited to the plasma membrane upon activation by DAG and calcium, directly interacting with membrane-bound substrates or channels.
Sensitivity to Cellular Conditions
The sensitivity of PKA and PKC to cellular conditions further differentiates them. PKA’s activity is tightly controlled by the concentration of cAMP, linking its activation to the presence of extracellular signals that influence cAMP levels. This makes PKA highly responsive to changes in the cell’s external environment.
PKC’s sensitivity, however, is more varied due to its diverse family of isoforms. Its activity can be influenced by factors such as the lipid composition of the cell membrane, which affects DAG availability, and the concentration of calcium ions. This enables PKC to respond to a broader range of cellular events and stresses.
Clinical Implications
The pivotal roles of PKA and PKC in regulating cellular processes extend to their involvement in various diseases and their potential as therapeutic targets.
Disease Associations
Role in Diseases and Disorders
Both PKA and PKC have been implicated in the pathogenesis of several diseases and disorders. Aberrations in PKA signaling have been linked to endocrine tumors, cardiac diseases, and neurodegenerative conditions. Similarly, dysregulation of PKC has been associated with cancer, diabetes, and neurological disorders. The complexity of these pathways often means that their malfunction can lead to a wide array of cellular and physiological abnormalities.
Therapeutic Targets
Drug Development
Given their central role in numerous critical pathways, both PKA and PKC represent attractive therapeutic targets. Drugs that modulate the activity of these kinases have the potential to treat a wide range of conditions. For PKA, targeting its regulatory subunits or the cAMP binding domain offers a strategy for modulating its activity in diseases where cAMP signaling is disrupted.
For PKC, the development of isoform-specific inhibitors is a key area of interest, given the diverse roles of different PKC isoforms in health and disease. Such specificity could allow for targeted therapies that mitigate the side effects often associated with broader kinase inhibitors.
Future Research Directions
The exploration into future research directions emphasizes the need for a deeper understanding of PKA and PKC signaling pathways, their interactions with other cellular components, and their roles in disease. Innovative approaches, such as the development of biomarkers for kinase activity and the use of gene editing technologies to investigate kinase function, are crucial. Additionally, the ongoing search for more selective and potent kinase inhibitors remains a high priority in the quest to translate these insights into therapeutic advances.
Frequently Asked Questions
What is phosphorylation?
Phosphorylation is a biochemical process in which a phosphate group is added to a protein or other organic molecule by a kinase. This modification can alter the activity, function, or location of the substrate, thereby playing a crucial role in regulating cellular processes such as signal transduction, metabolism, and cell cycle progression.
How do PKA and PKC differ in their activation?
PKA is activated by the accumulation of cyclic AMP (cAMP) within the cell, a molecule that signals the presence of extracellular cues like hormones. Conversely, PKC activation requires the presence of diacylglycerol (DAG) and calcium ions, signaling molecules that are often part of pathways responding to more diverse stimuli, including growth factors and immune signals.
Can PKA and PKC be therapeutic targets?
Yes, both PKA and PKC have been identified as potential therapeutic targets for various diseases, including cancer, cardiovascular diseases, and neurological disorders. By modulating the activity of these kinases, researchers aim to correct aberrant signaling pathways that contribute to disease pathology, offering new avenues for treatment development.
Conclusion
The intricate dance between Protein Kinase A and Protein Kinase C within the cellular landscape underscores the complexity of cellular signaling and its impact on health and disease. These kinases, through their distinct activation mechanisms and regulatory pathways, illustrate the precision with which cells navigate their internal and external environments, responding aptly to a myriad of signals.
Understanding the differences and interplays between PKA and PKC not only provides insight into cellular function but also opens up promising avenues for therapeutic interventions. As research continues to unravel the nuanced roles of these kinases, the potential for developing targeted treatments that modulate their activity grows, offering hope for combating diseases that stem from cellular signaling abnormalities.