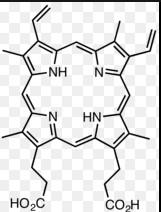
Porphyrins and protoporphyrins are complex organic compounds that play crucial roles in various biological processes, from oxygen transport in blood to photosynthesis in plants. These pigments are not just vital for life but also offer insights into medical diagnostics and treatments for numerous diseases. Their significance in both health and disease underscores their importance in biochemistry and medicine.
The difference between porphyrin and protoporphyrin lies primarily in their chemical structure and function. Porphyrins are a group of organic compounds with a distinctive ring-like structure, while protoporphyrins are a specific type of porphyrins that contain iron and are essential for the synthesis of hemoglobin. This distinction is crucial for understanding their roles in biological systems and their medical significance.
Porphyrins are synthesized in the body through a complex pathway that eventually leads to the creation of protoporphyrins when iron is incorporated into their structure. This process is fundamental to the production of hemoglobin, the protein in red blood cells responsible for oxygen transport. The synthesis and regulation of these compounds are essential for health, and disruptions can lead to various disorders, highlighting their biological importance.
Porphyrin Basics
Definition and Structure
Porphyrins are a group of organic compounds known for their ring-like structure. These molecules are built from four linked pyrrole rings through methine bridges, creating a large, stable macrocycle. This unique structure allows porphyrins to bind metals at their core, which is crucial for their biological functions.
Role in Nature
Porphyrins play a pivotal role in nature, acting as key components in various essential biological processes. For instance, they are central to the molecular makeup of hemoglobin and myoglobin, proteins responsible for oxygen transport and storage in animals. In plants, porphyrins are vital for chlorophyll, the compound that enables photosynthesis – the process by which plants convert light energy into chemical energy.
Common Types
Several types of porphyrins exist, each with specific roles and functions:
- Heme: Found in hemoglobin and myoglobin, essential for oxygen transport in blood.
- Chlorophyll: Enables photosynthesis in plants.
- Cytochromes: Involved in electron transport chains, crucial for cellular respiration.
Protoporphyrin Overview
Definition
Protoporphyrins are a specific subgroup of porphyrins that include a metal ion within their structure. The most common form, protoporphyrin IX, plays a critical role in the synthesis of heme, the iron-containing compound that gives hemoglobin its ability to bind and transport oxygen.
Structural Specifics
Protoporphyrins are characterized by their ability to chelate a metal ion, typically iron, within the center of their macrocyclic structure. This central metal ion is key to the molecule’s biological activity, especially in the formation of heme.
Biological Significance
The presence of protoporphyrin IX in the body is crucial for the synthesis of hemoglobin. Without protoporphyrin IX, the body cannot produce heme, leading to a reduction in hemoglobin’s effectiveness and potentially resulting in conditions like anemia.
Key Differences
Molecular Structure
While all porphyrins share a basic ring-like structure, protoporphyrins are distinguished by the inclusion of an iron ion. This addition is critical for the molecule’s function in oxygen transport and storage.
Biological Roles
Porphyrins are versatile and found in a variety of biological molecules, including chlorophyll and cytochromes. Protoporphyrins, however, are specifically important for their role in heme synthesis, directly impacting oxygen transport in the blood.
Occurrence and Sources
Porphyrins occur widely in nature, present in almost every living organism. They are synthesized in the body through a series of enzymatic reactions. Protoporphyrins, while also naturally occurring, are primarily found as intermediates in the pathway to heme synthesis in animals and humans.
Synthesis Pathways
Porphyrin Synthesis
The synthesis of porphyrins is a complex biochemical process that involves several steps, starting from simpler organic compounds like aminolevulinic acid (ALA). The body synthesizes ALA from glycine and succinyl-CoA, compounds found in the mitochondria. ALA then undergoes a series of enzymatic transformations, eventually forming the porphobilinogen molecule, which is the building block of porphyrins. Four molecules of porphobilinogen combine to form a hydroxymethylbilane, which then cyclizes to create the uroporphyrinogen III. This molecule undergoes further modifications, including decarboxylation and methylation, to produce various types of porphyrins, including protoporphyrin IX, the direct precursor to heme.
Conversion to Protoporphyrin
The conversion of uroporphyrinogen III into protoporphyrin IX is a pivotal step in porphyrin metabolism. This process involves several enzymatic steps that modify the side chains of the porphyrin ring, leading to the synthesis of coproporphyrinogen III and then protoporphyrinogen IX. The final step in the conversion process involves the oxidation of protoporphyrinogen IX to protoporphyrin IX, which is then ready to bind with iron to form heme. This step is catalyzed by the enzyme protoporphyrinogen oxidase, and its efficiency is critical for the proper synthesis of hemoglobin and other heme-containing proteins.
Applications and Implications
Medical Significance
Porphyrins and protoporphyrins have profound medical significance. The ability of porphyrins to bind metals and participate in oxygen transport makes them crucial in diagnosing and treating blood disorders. Abnormal levels of porphyrins and their precursors can indicate diseases such as porphyria and anemia. Moreover, protoporphyrin IX, a specific type of porphyrin, is used in photodynamic therapy for treating cancer. This therapy involves administering a porphyrin-based compound that accumulates in cancer cells. When exposed to light, it produces reactive oxygen species that kill the cancer cells.
Industrial Applications
Beyond their biological and medical importance, porphyrins have industrial applications as well. Their photocatalytic properties make them useful in environmental cleanup efforts, such as breaking down pollutants. Porphyrins are also used in the development of organic light-emitting diodes (OLEDs) and solar cells, contributing to the advancement of renewable energy technologies.
Research Areas
Research on porphyrins and protoporphyrins spans multiple disciplines. Scientists are exploring their use in nanotechnology for creating more efficient drug delivery systems. In biochemistry, research focuses on understanding their role in cellular processes and how alterations in their synthesis contribute to diseases. Additionally, in material science, porphyrins are investigated for their potential in sensing technologies and catalysis, showcasing their versatility across scientific fields.
Challenges and Solutions
Synthesis Challenges
Synthesizing porphyrins in the lab presents challenges, primarily due to their complex structure and the need for precise control over the metal ion incorporation. However, advancements in chemical synthesis techniques have led to more efficient methods, including the use of template-directed synthesis and biomimetic approaches. These methods mimic the natural synthesis pathways, allowing for the production of porphyrins with specific characteristics tailored for various applications.
Detection and Measurement
Detecting and measuring porphyrins in biological samples is essential for diagnosing disorders related to their metabolism. The main challenge lies in the low concentrations at which these compounds are present and their sensitivity to light and oxidation. Solutions include the development of highly sensitive and specific spectroscopic methods. Techniques such as mass spectrometry and liquid chromatography have improved the accuracy and efficiency of porphyrin detection, enabling earlier diagnosis and better monitoring of diseases.
Frequently Asked Questions
What are porphyrins?
Porphyrins are organic compounds characterized by a unique structure composed of four modified pyrrole subunits interconnected at their α carbon atoms via methine bridges. This ring structure is crucial for their ability to bind metals and perform various biological functions, including serving as the core of heme, vital for oxygen transport and cell respiration.
How are protoporphyrins different from other porphyrins?
Protoporphyrins differ from other porphyrins by the presence of iron within their molecular structure, making them a key component in the formation of heme, the non-protein part of hemoglobin. This distinction is critical for their role in oxygen transport and storage in living organisms, setting them apart from other porphyrins that may not directly participate in these processes.
Why are porphyrins important in medicine?
Porphyrins are essential in medicine due to their involvement in diagnosing and treating a range of diseases, including porphyrias and anemias. Their unique absorption and fluorescence properties are utilized in photodynamic therapy for cancer treatment, highlighting their significance in medical diagnostics and therapeutic strategies.
How do porphyrins and protoporphyrins impact health?
Porphyrins and protoporphyrins impact health through their roles in the synthesis of hemoglobin and other heme-containing enzymes, which are crucial for oxygen transport and cellular metabolism. Imbalances or defects in their synthesis can lead to various health issues, including porphyria and anemia, underscoring their importance in maintaining physiological functions.
Conclusion
Porphyrins and protoporphyrins, with their complex structures and critical roles in biological processes, underscore the intricate connections between organic chemistry and life sciences. Their distinction, while subtle, has profound implications for our understanding of physiological processes and the development of medical diagnostics and treatments. This insight not only enriches our knowledge of biochemistry but also opens avenues for advancing medical science and improving health outcomes.
The exploration of porphyrins and protoporphyrins reveals the beauty and complexity of nature’s design in sustaining life. By understanding their differences and functions, we gain valuable perspectives on their roles in health and disease, showcasing the importance of these molecules in the fabric of biological systems. This knowledge encourages further research and innovation, aiming to harness their potential for the betterment of human health.