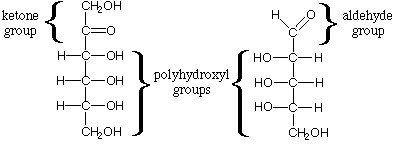
Carbohydrates are fundamental to life on Earth, serving as energy sources, structural components, and participants in various biological processes. Among their diverse forms, polyhydroxy aldehydes and polyhydroxy ketones stand out for their roles in both simple sugars and complex polysaccharides. These compounds, rich in hydroxyl groups, are pivotal in the biochemistry of living organisms and industrial applications, highlighting the versatility and complexity of carbohydrates.
Polyhydroxy aldehydes and polyhydroxy ketones are types of carbohydrates that differ mainly in their functional groups: aldehydes and ketones, respectively. Polyhydroxy aldehydes, known as aldoses, have an aldehyde group at one end of the molecule. In contrast, polyhydroxy ketones, or ketoses, feature a ketone group within their carbon chain. This distinction influences their chemical behavior, structure, and role in biological systems.
The relevance of these compounds extends beyond their chemical classification. In biological systems, they are involved in energy storage and retrieval, signaling, and structural integrity. Industrially, they find applications ranging from food production to pharmaceuticals. The comparison between polyhydroxy aldehydes and ketones unveils a fascinating aspect of organic chemistry’s impact on both nature and technology, highlighting the interconnectedness of chemical structure and functional efficacy.
Core Concepts
Definition of Carbohydrates
Carbohydrates are organic molecules that consist of carbon (C), hydrogen (H), and oxygen (O), usually with a hydrogen:oxygen atom ratio of 2:1, similar to water (H₂O). They are one of the primary types of nutrients found in foods and serve as a major energy source for the body. Beyond energy, carbohydrates play critical roles in the structure of cells and tissues, and are involved in various biological processes such as cell signaling and immune response.
Polyhydroxy Aldehydes
Characteristics
Polyhydroxy aldehydes, or aldoses, are carbohydrates that contain an aldehyde group (-CHO) at the end of the carbon chain. This group makes them highly reactive. They can exist in both linear and ring forms, with the ring form being more prevalent in biological systems. Common examples include glucose and galactose, essential sugars in human metabolism.
Examples
- Glucose: Often called blood sugar, it’s a primary energy source for cells.
- Galactose: Part of lactose, the sugar found in milk, and essential for energy production.
Polyhydroxy Ketones
Characteristics
Polyhydroxy ketones, or ketoses, feature a ketone group (>C=O) within their carbon skeleton. This structural element renders them slightly less reactive than aldoses. Like aldoses, ketoses can also form ring structures when dissolved in water, which is common in biological systems. Fructose is a well-known ketose, recognized for its sweetness.
Examples
- Fructose: A sugar found in fruits and honey, noted for being sweeter than glucose.
- Ribulose: A pentose ketose involved in the photosynthesis process of plants.
Structural Differences
Functional Groups
The primary difference between polyhydroxy aldehydes and ketones lies in their functional groups. An aldehyde group at the carbon chain’s end characterizes aldoses, making them more prone to oxidation. Ketones have a ketone group located within the carbon chain, which affects their reactivity and chemical properties differently.
Molecular Structure
The molecular structure of these compounds significantly impacts their physical and chemical properties. Aldoses and ketoses can form five- or six-membered rings in aqueous solutions, a phenomenon known as cyclization. This structural variation influences their solubility, reactivity, and biological functionality.
Role in Biology
Polyhydroxy Aldehydes
Aldoses play vital roles in biology, notably glucose, the body’s key energy source. These molecules are integral to cellular respiration, where glucose breaks down to produce ATP (adenosine triphosphate), the energy currency of the cell. They also serve as building blocks for larger molecules like starch and cellulose.
Polyhydroxy Ketones
Ketoses, particularly fructose, are crucial in metabolism and energy production. Fructose is metabolized by the liver and can contribute to the synthesis of glycogen, the storage form of glucose. Ketoses also play roles in the pentose phosphate pathway, a metabolic process that generates NADPH and riboses.
Chemical Behavior
Reactivity
The reactivity of these compounds is influenced by their functional groups. Aldoses are generally more reactive due to their aldehyde group, making them susceptible to oxidation reactions. Ketones, with their carbonyl group located within the carbon chain, are less prone to such reactions but can undergo keto-enol tautomerism, which has implications in their biological activity.
Solubility and Melting Points
The solubility of polyhydroxy aldehydes and ketones in water is a key property, attributed to their numerous hydroxyl groups that can form hydrogen bonds with water molecules. Generally, these compounds are highly soluble in water. Their melting points can vary but are influenced by molecular weight and structural conformation, with those forming stronger hydrogen bonds typically having higher melting points.
Applications
Industrial Use
Polyhydroxy aldehydes and ketones are cornerstones in industrial applications due to their versatile chemical properties. They serve as key ingredients in the manufacture of plastics, resins, and textiles, showcasing their integral role in material science. For instance, the polymerization of certain aldoses can lead to biodegradable plastics, marking a significant step towards sustainable materials.
In the realm of food industry, these compounds, especially fructose, are prized for their sweetening properties. High-fructose corn syrup, a widely used sweetener in beverages and processed foods, exemplifies the industrial use of ketoses. Moreover, their role as flavor enhancers and preservatives underscores their indispensable value in food science and technology.
Health and Medicine
In healthcare, polyhydroxy aldehydes and ketones are pivotal in diagnostic procedures and treatment formulations. Glucose, for instance, is fundamental in the management and monitoring of diabetes, with blood sugar levels serving as crucial indicators of patient health. Furthermore, these carbohydrates are involved in the synthesis of various pharmaceuticals, highlighting their significance in drug development.
Their role extends to nutrition, where understanding the metabolism of these sugars is essential for dietary planning and managing metabolic disorders. Additionally, certain polyhydroxy aldehydes and ketones are used in cosmetics for their moisturizing and humectant properties, illustrating their broad applicability in health and wellness.
Synthesis and Isolation
Laboratory Synthesis
The synthetic pathways to produce polyhydroxy aldehydes and ketones involve several key methods, highlighting the advancements in organic chemistry. Chemical synthesis often employs aldol condensation or the Kiliani-Fischer synthesis, allowing for the precise creation of these compounds with desired configurations. Additionally, enzymatic synthesis presents a more selective and eco-friendly approach, utilizing biocatalysts to facilitate the formation of specific stereoisomers.
Natural Occurrence and Extraction
Polyhydroxy aldehydes and ketones are abundant in nature, predominantly found in plants and fruits. Their extraction from natural sources involves processes like hydrolysis of polysaccharides for aldoses and the separation of fructose from sources rich in sucrose. Techniques such as chromatography and crystallization are employed to purify these compounds, ensuring their availability for both research and industrial application.
Future Directions
Research Trends
Current research in the field of polyhydroxy aldehydes and ketones is geared towards exploring their biological functions and therapeutic potentials. Studies on the role of these sugars in cell signaling and disease mechanisms are paving the way for novel treatments. Moreover, the environmental impact of synthesizing and utilizing these compounds is under scrutiny, driving the search for sustainable methods of production.
Emerging research focuses on the genetic engineering of microorganisms to enhance the yield and efficiency of carbohydrate production. This biotechnological approach promises to revolutionize the way we produce and utilize these essential compounds, aligning with goals of sustainability and efficiency.
Technological Advancements
Innovation in the production and use of polyhydroxy aldehydes and ketones is evident in the development of new catalytic processes that increase synthesis efficiency while reducing environmental impact. Furthermore, advancements in nanotechnology are employing these carbohydrates in the creation of smart materials and drug delivery systems, showcasing their potential in cutting-edge applications.
The integration of computational biology and chemical engineering is facilitating the design of processes that optimize the production and application of these compounds. As research progresses, we can anticipate a future where the synthesis, utilization, and sustainability of polyhydroxy aldehydes and ketones are significantly enhanced, marking a new era of biochemical innovation and industrial application.
Frequently Asked Questions
What are carbohydrates?
Carbohydrates are organic compounds made up of carbon, hydrogen, and oxygen, typically with a hydrogen:oxygen atom ratio of 2:1, similar to water. They are one of the four essential macromolecules of life, serving as key energy sources, structural components, and cellular messengers. Carbohydrates are classified based on their structure and complexity into simple sugars (monosaccharides and disaccharides) and complex carbohydrates (oligosaccharides and polysaccharides).
How do polyhydroxy aldehydes differ from polyhydroxy ketones?
Polyhydroxy aldehydes and polyhydroxy ketones differ primarily in their functional groups. Polyhydroxy aldehydes contain an aldehyde group (-CHO) at one end of their carbon chain, making them aldoses. Polyhydroxy ketones possess a ketone group (>C=O) within the carbon chain, classifying them as ketoses. This distinction affects their chemical reactivity, structure, and biological roles, with each playing unique roles in metabolism and industrial applications.
Why are polyhydroxy aldehydes and ketones important in biology?
Polyhydroxy aldehydes and ketones are crucial in biology due to their roles in energy storage, metabolic pathways, and structural components of cells. They are involved in key processes such as glycolysis and the Calvin cycle, contributing to the production of ATP, the energy currency of the cell. Additionally, they serve as building blocks for more complex carbohydrates, which are essential for cell structure and function, including cell-cell recognition and signaling.
What are the industrial applications of these compounds?
Polyhydroxy aldehydes and ketones have wide-ranging industrial applications, from food sweeteners and biofuels to pharmaceuticals and cosmetics. Their chemical properties make them valuable as intermediates in synthetic chemistry, in the production of biodegradable plastics, and as solvents. In the food industry, they are used to enhance flavor and extend shelf life, while in medicine, they play roles in drug formulation and as diagnostic agents.
Conclusion
The exploration of polyhydroxy aldehydes and polyhydroxy ketones unveils a rich landscape of chemistry that intersects with biology, industry, and technology. These compounds exemplify the intricate ways in which molecular structure can influence physical properties, chemical reactivity, and biological functionality. Their diversity and versatility underscore the complexity of organic compounds and their pivotal roles in sustaining life and advancing human technology.
Understanding the differences and similarities between polyhydroxy aldehydes and ketones not only enriches our knowledge of organic chemistry but also highlights the potential for new discoveries and applications. As research progresses, these compounds will undoubtedly continue to play significant roles in various fields, from healthcare to sustainable energy, demonstrating the enduring importance of studying and harnessing the power of chemical structures.