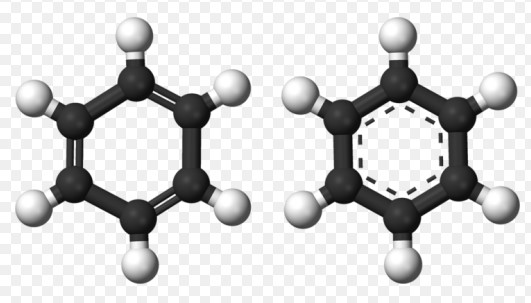
Aromatic hydrocarbons are a fascinating group of compounds that play a pivotal role in both our natural and industrial worlds. These organic molecules are characterized by their stable ring structures, which are not only a cornerstone of organic chemistry but also a key component in various products and processes. From the fuels that power our vehicles to the pharmaceuticals that maintain our health, the significance of aromatic hydrocarbons is widespread and undeniable.
The difference between polycyclic and polynuclear aromatic hydrocarbons lies primarily in their structures and formation processes. Polycyclic aromatic hydrocarbons (PAHs) consist of multiple interconnected aromatic rings without substituents, whereas polynuclear aromatic hydrocarbons contain two or more aromatic rings that are fused together or linked by a bond, potentially including side chains or substituents.
The environmental and health implications of these compounds are substantial, with both types posing various degrees of risk. PAHs, in particular, are notorious for their carcinogenic properties, highlighting the importance of understanding their characteristics, sources, and effects. This knowledge is not only essential for chemists but also for environmental scientists and health professionals who are on the frontline of addressing the challenges posed by these persistent pollutants.
Basics of Aromatic Hydrocarbons
Aromatic Hydrocarbon Properties
Aromatic hydrocarbons are a class of compounds known for their stable, resonant ring structures. This stability comes from a phenomenon known as resonance, where electrons are shared across the entire structure, rather than being confined to a single bond. This electron distribution contributes to the chemical stability of these compounds, making them less reactive compared to their aliphatic counterparts. The most iconic example of an aromatic hydrocarbon is benzene, with its six carbon atoms forming a ring, each bonded to a hydrogen atom.
Types of Aromatic Hydrocarbons
Aromatic hydrocarbons can be broadly categorized into single-ring and multi-ring varieties. Single-ring aromatics, like benzene, are the simplest form. Multi-ring aromatic hydrocarbons, on the other hand, include structures where two or more rings are connected. These can be further divided into polycyclic and polynuclear aromatic hydrocarbons, each with distinct characteristics and implications.
Polycyclic Aromatic Hydrocarbons (PAHs)
Definition and Structure
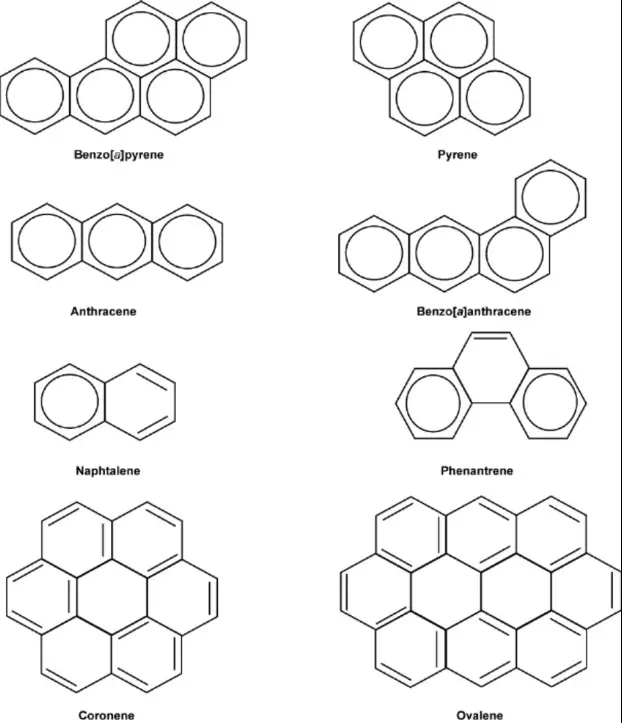
Polycyclic Aromatic Hydrocarbons (PAHs) are compounds composed of multiple aromatic rings fused together. These structures do not have aliphatic chains or atoms interrupting the fused rings, making them purely aromatic. PAHs are common in the environment and can form complex structures. For example, naphthalene, the simplest PAH, consists of two benzene rings fused together, while benzo[a]pyrene, a more complex PAH, consists of five benzene rings in a specific arrangement.
Formation and Sources
PAHs are formed primarily through the incomplete combustion of organic material. This can occur naturally, such as during forest fires or volcanic activity, or through human activities like burning fossil fuels, tobacco smoke, and the charbroiling of meat. Industrial processes, including the production of coke and tar, also contribute to PAH emissions.
Environmental Impact and Health Risks
PAHs are of significant concern due to their carcinogenic properties. They can bind to DNA, causing mutations that lead to cancer. The environmental impact of PAHs is also profound, as they can persist in soil and water, affecting ecosystems. Notably, PAHs have been linked to respiratory problems and skin irritations in humans, and their presence in the environment can lead to bioaccumulation in wildlife, posing risks up the food chain.
Polynuclear Aromatic Hydrocarbons
Definition and Distinction
Polynuclear Aromatic Hydrocarbons differ from PAHs in that they may include non-fused rings or side chains. These compounds can have two or more aromatic rings that are connected but not necessarily fused, allowing for a greater variety of structures. This distinction is crucial for understanding the behavior and effects of these compounds.
Formation Mechanisms
Polynuclear aromatic hydrocarbons are formed through similar processes as PAHs, including natural events and human activities. However, their specific structures can be influenced by the conditions under which they are created, such as temperature and the presence of catalysts. Chemical reactions, such as alkylation, can also play a role in the formation of polynuclear aromatic hydrocarbons, leading to a diversity of compounds with varying properties and behaviors.
Health and Environmental Considerations
The health and environmental impacts of polynuclear aromatic hydrocarbons can be as significant as those of PAHs. Their toxicity and persistence in the environment make them a concern for both human health and ecological systems. The potential for these compounds to cause cancer, respiratory issues, and other health problems necessitates ongoing research and monitoring to understand their full effects and to develop strategies for mitigation and remediation.
Key Differences Explained
Structural Differences
The distinction between polycyclic and polynuclear aromatic hydrocarbons centers on their molecular architecture. Polycyclic aromatic hydrocarbons (PAHs) are composed of two or more aromatic rings that are fused together, forming a single, interconnected structure. This fusion results in a molecule where the rings share carbon atoms. On the other hand, polynuclear aromatic hydrocarbons may also consist of multiple aromatic rings, but these rings are not necessarily fused. They can be connected by a single bond or even include additional atoms or side chains that do not disrupt the aromatic system.
For instance, in naphthalene, a polycyclic compound, two benzene rings are fused, sharing two carbon atoms. In contrast, biphenyl, which can be considered a simple polynuclear aromatic hydrocarbon, consists of two benzene rings connected by a single bond, with no shared carbons between the aromatic rings.
Formation and Sources
The formation of PAHs and polynuclear aromatic hydrocarbons can occur under various conditions, both natural and anthropogenic. PAHs are predominantly generated through the incomplete combustion of carbon-containing materials, such as fossil fuels, wood, and other biomass. This process can occur in both controlled environments, like industrial settings, and uncontrolled situations, such as wildfires.
Polynuclear aromatic hydrocarbons, while also produced through similar combustion processes, can form through more varied chemical reactions, including the degradation of larger PAHs or through synthetic routes in industrial chemical processes. The sources of polynuclear aromatic hydrocarbons, therefore, extend beyond combustion, including chemical manufacturing and processing industries.
Health and Environmental Impact
Both PAHs and polynuclear aromatic hydrocarbons pose significant health risks and environmental hazards. However, the degree of impact and the mechanisms through which they affect living organisms and ecosystems can vary. PAHs, with their stable, fused-ring structures, are known to be highly persistent in the environment, capable of accumulating in the food chain and posing long-term health risks, including cancer, respiratory disorders, and skin diseases.
Polynuclear aromatic hydrocarbons, while also toxic, may exhibit different environmental behaviors and health effects due to their structural diversity. Their toxicity can depend on the specific arrangement of the rings and the presence of functional groups or substituents, which can influence their reactivity and persistence in the environment.
Detection and Analysis
Techniques for Identification
Identifying and quantifying PAHs and polynuclear aromatic hydrocarbons in environmental samples is crucial for monitoring their presence and understanding their impact. Advanced analytical techniques are employed for this purpose, including:
- Gas chromatography (GC) coupled with mass spectrometry (MS) for separating and identifying compounds based on their mass-to-charge ratio.
- High-performance liquid chromatography (HPLC) used for analyzing samples that are not suitable for GC due to their high boiling points or instability.
- Spectroscopic methods, such as UV-visible spectroscopy and fluorescence spectroscopy, for rapid screening of samples based on the unique absorption and emission characteristics of aromatic compounds.
Challenges in Differentiation
Differentiating between PAHs and polynuclear aromatic hydrocarbons in mixed environmental samples presents challenges. The structural similarities among these compounds mean that they can exhibit similar physical and chemical properties, making selective identification difficult. Furthermore, the complexity of environmental samples, which may contain a vast array of similar compounds, necessitates the use of sophisticated, high-resolution analytical techniques and careful interpretation of data to accurately distinguish between these two types of hydrocarbons.
Mitigation and Management
Reducing Exposure
Minimizing human exposure to harmful PAHs and polynuclear aromatic hydrocarbons involves both regulatory measures and personal precautions. On a regulatory level, governments and international bodies can enact and enforce policies that limit emissions from industrial and vehicular sources, promote the use of cleaner energy sources, and establish guidelines for safe levels of exposure. Individuals can reduce their risk of exposure by:
- Avoiding smoking and secondhand smoke.
- Choosing grilled or smoked foods wisely, as these cooking methods can generate PAHs.
- Using proper protective equipment when working in environments where exposure to these compounds is likely.
Environmental Cleanup Efforts
Efforts to remove or degrade PAHs and polynuclear aromatic hydrocarbons from polluted sites focus on both physical methods and biological processes. Bioremediation techniques, which employ microorganisms to break down pollutants, have shown promise in degrading these compounds into less harmful substances. Additionally, chemical degradation methods, including advanced oxidation processes, can directly break down the hydrocarbons using chemical agents.
FAQs
What are Aromatic Hydrocarbons?
Aromatic hydrocarbons are organic compounds comprised of carbon and hydrogen with one or more planar ring structures exhibiting resonance. This category includes both simple single-ring molecules and complex multi-ring structures. These compounds are known for their stability and are extensively used in various industries, including pharmaceuticals, dyes, and plastics.
How do PAHs affect human health?
Polycyclic aromatic hydrocarbons (PAHs) can have significant adverse effects on human health, primarily when exposure occurs over prolonged periods. These compounds are linked to various forms of cancer, respiratory issues, and skin irritations. The carcinogenic potential of PAHs is particularly concerning, emphasizing the need for effective exposure control and regulatory measures.
Are Polynuclear Aromatic Hydrocarbons different from PAHs?
Yes, polynuclear aromatic hydrocarbons differ from polycyclic aromatic hydrocarbons (PAHs) in structure and formation. Polynuclear variants contain multiple aromatic rings that can be either fused together or linked without the necessity of forming a single, contiguous system. This distinction is crucial for understanding their chemical behavior, environmental persistence, and potential health impacts.
How can exposure to PAHs be minimized?
Minimizing exposure to PAHs involves a combination of regulatory strategies and personal precautions. Industries can implement cleaner combustion processes and adhere to environmental regulations to reduce PAH emissions. Individuals can avoid smoking, limit consumption of charred foods, and use proper protective gear when exposed to PAHs during occupational activities.
Conclusion
The intricacies of polycyclic and polynuclear aromatic hydrocarbons extend far beyond their molecular structures, touching upon aspects of environmental science, public health, and industrial practices. The differences between these compounds underscore the complexity of managing their presence in our environment and the importance of ongoing research to fully understand their impacts. By shedding light on these distinctions, we pave the way for more informed decisions in environmental policies and health guidelines.
As we continue to explore and mitigate the effects of aromatic hydrocarbons, the collective efforts of scientists, industry stakeholders, and policymakers are crucial. Recognizing the unique challenges posed by both polycyclic and polynuclear aromatic hydrocarbons is a step towards safeguarding our environment and health, underscoring the significance of chemical knowledge in addressing contemporary issues.