Phosgene and diphosgene are two chemical compounds historically used in warfare and various industrial processes. Although both are notorious for their use as chemical weapons during World War I, they have distinct properties and applications that set them apart. This differentiation is crucial for specialists in fields ranging from historical research to modern industrial applications.
Phosgene is a colorless gas under ambient conditions, known chemically as carbonyl dichloride. On the other hand, diphosgene appears as a colorless liquid, which is more stable and easier to handle than phosgene. While both chemicals are toxic, diphosgene’s ability to convert to phosgene upon contact with moisture makes it particularly hazardous.
The significance of understanding the differences between these two chemicals extends beyond their historical use as warfare agents. It encompasses their impact on environmental safety, industrial usage, and the stringent regulations surrounding their handling and disposal. This knowledge not only ensures compliance with safety standards but also guides the development of safer chemical practices.
Historical Context
Discovery and Early Use of Phosgene
Phosgene, known chemically as carbonyl dichloride, was first synthesized in 1812 by John Davy. Its initial applications were not in the realm of warfare but in the chemical industry. However, its role dramatically shifted during World War I. Recognized for its potential as a lethal chemical weapon, phosgene was extensively used by both sides to break the stalemate of trench warfare. It became infamous for its delayed-action effects, which often resulted in casualties long after exposure.
Development of Diphosgene
Building on the deadly legacy of phosgene, chemists developed diphosgene in the later stages of World War I. Diphosgene was designed to be a more stable and easier-to-handle liquid form of phosgene. This new compound could be stored and deployed more effectively on the battlefield, providing a tactical advantage by reducing the risks associated with gas deployment and increasing the ease of use in various combat scenarios.
Chemical Properties
Phosgene
Chemical Structure and Formula
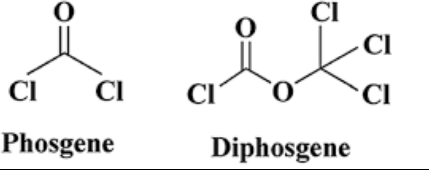
The chemical structure of phosgene is represented by the formula COCl₂. It consists of a carbon atom double-bonded to an oxygen atom and two chlorine atoms. This simple structure belies its reactivity and toxicity, which have made it a significant compound in both industrial and military contexts.
Physical Characteristics
Phosgene is a colorless gas at room temperature with a suffocating odor often likened to musty hay. It is highly volatile, with a boiling point of 8.2 degrees Celsius, which allows it to disperse quickly when released in the air.
Diphosgene
Chemical Structure and Formula
Diphosgene shares a similar chemical makeup to phosgene but has an additional complexity in its structure, represented by the formula ClCO₂CCl₃. This structure makes it a liquid at room temperature, which significantly alters its handling and storage requirements.
Physical Characteristics
Unlike phosgene, diphosgene is a colorless liquid under normal conditions. It has a higher boiling point of 128 degrees Celsius, which contributes to its stability and ease of handling compared to its gaseous counterpart.
Production Methods
Phosgene Production
Industrial Synthesis Techniques
Phosgene is typically produced through the reaction of carbon monoxide and chlorine gas in the presence of a charcoal catalyst at high temperatures. This method has been refined over the decades to enhance yield and reduce risks:
- Controlled Environment: Conducting the synthesis in a controlled environment minimizes the escape of phosgene gas, ensuring the safety of the production facility.
- Temperature Management: Careful management of reaction temperatures prevents the decomposition of reactants, improving efficiency.
Diphosgene Production
Synthesis and Improvements over Phosgene
The synthesis of diphosgene from phosgene involves a reaction with triphosgene under controlled conditions. This process has been optimized to reduce the hazards associated with phosgene gas:
- Use of Intermediates: By using safer intermediates, the direct handling of large volumes of phosgene gas is minimized.
- Advanced Containment: Modern containment techniques prevent accidental releases, enhancing workplace safety.
Toxicity Levels
Exposure to Phosgene
Mechanisms of Toxicity
Phosgene poisons the pulmonary system by reacting with the water in lung tissue, forming hydrochloric acid and carbon dioxide. This reaction leads to severe irritation and pulmonary edema.
Symptoms of Exposure
The symptoms of phosgene exposure include:
- Breathing Difficulty: Shortness of breath and coughing are immediate signs of exposure.
- Chest Pain: Severe irritation can cause long-lasting chest pain.
- Delayed Effects: Symptoms can take hours to manifest, often complicating diagnosis and treatment.
Exposure to Diphosgene
Comparative Mechanisms of Toxicity
Diphosgene offers the same toxic effects as phosgene but with an added risk due to its liquid state. It can penetrate clothing and convert to phosgene upon contact with moisture on the skin or in the lungs.
Symptoms and Immediate Effects
Similar to phosgene, the symptoms of diphosgene exposure are delayed and include severe respiratory distress. However, its ability to remain on surfaces can cause prolonged exposure risks, making immediate decontamination critical.
Safety and Handling
Phosgene Protocols
Safety Measures in Industrial Use
Handling phosgene requires strict safety protocols due to its high toxicity and potential for severe health impacts. Key safety measures include:
- Proper Ventilation: Ensuring well-ventilated areas to disperse any accidental leaks.
- Protective Gear: Wearing appropriate respiratory protection and protective clothing to prevent any direct contact with the gas.
- Leak Detection Systems: Installing sensitive detection systems that can alert workers to the presence of phosgene even at low concentrations.
Emergency Response
In case of a phosgene leak or exposure, quick and effective emergency response is crucial:
- Immediate Evacuation: Ensuring all personnel evacuate the affected area swiftly.
- Decontamination: Exposed individuals must undergo thorough decontamination procedures.
- Medical Attention: Providing immediate medical attention to those showing symptoms of exposure.
Diphosgene Protocols
Handling Improvements
Diphosgene’s liquid form allows for safer handling procedures compared to phosgene. Improvements include:
- Sealed Containers: Using double-sealed containers to prevent leaks.
- Temperature Control: Maintaining storage areas at specific temperatures to stabilize the compound.
Safety Measures and Emergency Response
Similar to phosgene, but with adjustments due to its physical state:
- Rapid Containment: Quickly containing spills using absorbent materials that neutralize chemicals.
- Skin Protection: Using gloves and protective suits to avoid skin contact, as diphosgene can penetrate standard materials more easily.
Applications and Uses
Industrial Uses of Phosgene
Key Applications in Manufacturing
Phosgene is a critical component in the manufacture of:
- Polycarbonates and Polyurethanes: These are used in everything from electronics to automotive parts and building materials.
- Pharmaceuticals: Synthesizing key intermediates for certain medications.
Industrial Uses of Diphosgene
Benefits Over Phosgene in Specific Applications
Diphosgene offers several advantages, making it preferable in some industrial processes:
- Safer Handling: As a liquid, it can be more easily controlled and transferred with reduced risk of accidental inhalation.
- Efficiency in Synthesis: Used in the synthesis of pharmaceuticals where its stability improves overall safety and reaction control.
Environmental Impact
Phosgene Impact
Environmental Risks and Mitigation
Phosgene’s toxicity poses significant environmental risks, particularly in the event of industrial accidents. Mitigation strategies include:
- Strict Emission Controls: Implementing technologies to capture and neutralize phosgene before it can be released into the atmosphere.
- Emergency Response Plans: Preparing for and mitigating potential spills to reduce environmental impact.
Diphosgene Impact
Comparative Environmental Considerations
While diphosgene shares many of the same risks as phosgene, its liquid state allows for different mitigation techniques:
- Spill Containment Systems: Specialized containment solutions are required to manage liquid spills effectively.
- Waste Treatment: Enhanced treatment methods to ensure that residues do not persist in the environment.
Legal and Ethical Considerations
Regulations Governing Use and Handling
Both phosgene and diphosgene are subject to rigorous international regulations that govern their use, storage, and transportation, including:
- Chemical Weapons Convention (CWC): This treaty restricts the production and stockpiling of chemical weapons.
- Local Environmental Laws: Various countries have specific regulations designed to protect workers and the environment from chemical exposures.
Ethical Issues in Chemical Production
Producing and handling hazardous chemicals raises significant ethical concerns:
- Safety First: Prioritizing the health and safety of workers and communities.
- Informed Consent: Ensuring that all personnel are fully informed of the risks involved.
Future Prospects
Research Trends in Chemical Safety
Recent research focuses on developing safer chemical processes and finding safer alternatives to toxic chemicals like phosgene and diphosgene.
Potential Alternatives and Innovations
Innovations include:
- Safer Chemicals: Developing less hazardous chemicals that can perform the same industrial functions.
- Advanced Safety Equipment: Enhancing protective gear and detection systems to reduce exposure risks.
Frequently Asked Questions
What is Phosgene?
Phosgene, chemically known as COCl₂, is a toxic gas with a suffocating odor, historically used as a chemical weapon. It is primarily used industrially to produce pharmaceuticals and pesticides.
What is Diphosgene?
Diphosgene is a chemical compound similar to phosgene but exists as a liquid at room temperature, making it easier to handle. It serves as an intermediate in chemical synthesis and a warfare agent.
How are Phosgene and Diphosgene different?
Phosgene is a gas at room temperature, whereas diphosgene is a liquid. This state difference affects their handling and storage. Diphosgene also has a slightly higher boiling point and can generate phosgene when it comes into contact with moisture.
What are the uses of Phosgene today?
Today, phosgene is primarily used in the manufacture of plastics, pharmaceuticals, and agrochemicals. Its reactive nature makes it valuable in synthesizing polycarbonates and polyurethanes.
What safety measures are necessary when handling Phosgene?
Handling phosgene requires strict safety protocols, including the use of protective gear, proper ventilation, and emergency readiness due to its highly toxic nature.
Conclusion
The exploration of phosgene and diphosgene reveals more than their roles in history; it opens a window into the complexities of chemical safety and industrial application. The careful distinction between these compounds underscores the importance of chemical literacy in preventing hazards and promoting safe practices.
Understanding these chemicals’ properties, uses, and risks helps ensure those handling them are well-prepared to mitigate their potential dangers. As the industrial and ethical landscape evolves, the knowledge of such substances will remain crucial for safety and compliance in various sectors.

