In the world of chemistry and environmental science, the terms percent abundance and relative abundance are crucial for understanding the composition and distribution of elements and isotopes within a sample. These concepts are foundational in the analysis of chemical substances and play a pivotal role in various scientific and industrial applications, from dating archaeological finds to monitoring ecological changes.
Percent abundance refers to the proportion of a specific isotope of an element relative to all its isotopes, expressed as a percentage. Relative abundance, on the other hand, measures how common or rare an isotope is compared to others within the same context, but it is not expressed in percentage terms. Both measures offer insights into the elemental makeup of substances, but they serve different analytical purposes.
Distinguishing between percent abundance and relative abundance is essential for accurate data interpretation in scientific research. These concepts aid in the precise determination of elemental and isotopic compositions, which are critical for applications ranging from understanding ancient climates to tracking modern-day environmental pollutants. This understanding helps scientists make informed decisions and draw accurate conclusions in their respective fields.
Basics of Abundance
Atomic Structure
Atoms, the building blocks of matter, consist of a nucleus surrounded by electrons. The nucleus contains protons and neutrons, with the number of protons defining the element. However, the number of neutrons can vary among atoms of the same element, leading to different isotopes. Isotopes have the same chemical properties but differ in physical properties, such as mass.
Explanation of Isotopes
Isotopes are variations of elements that have the same number of protons but a different number of neutrons. This difference does not affect the chemical behavior of the atom but does influence its atomic mass and stability. Some isotopes are stable, while others are radioactive, decaying over time into other elements.
Defining Abundance
Percent Abundance
Percent abundance refers to the proportion of a particular isotope relative to all isotopes of that element in a sample, expressed as a percentage. It is crucial for determining the average atomic mass of an element.
Relative Abundance
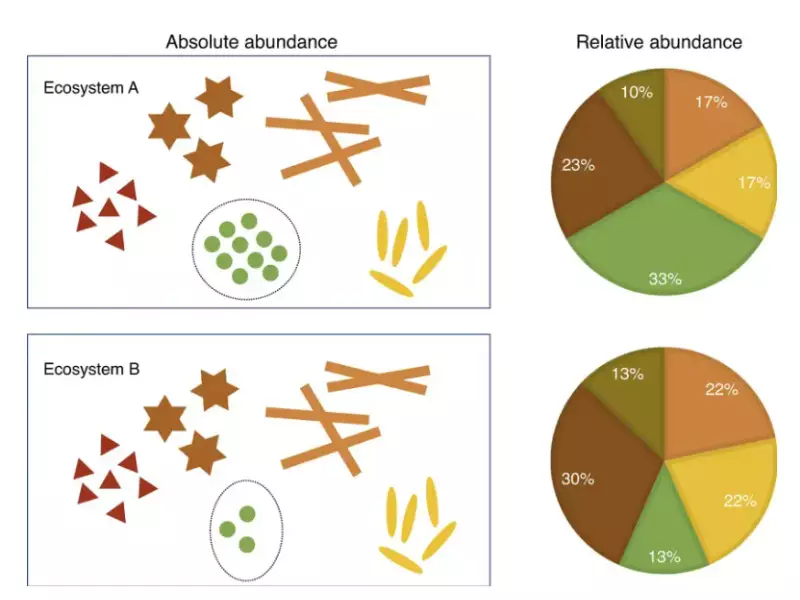
Relative abundance, on the other hand, measures the ratio of the quantity of a specific isotope to the total isotopes of that element. It is often used in ecology and environmental science to study population dynamics and biodiversity.
Percent Abundance
Concept Overview
Percent abundance provides insight into the most prevalent isotopes of an element in nature. This information is essential for calculating the average atomic mass of elements as listed on the periodic table, a weighted average considering the mass and percent abundance of each isotope.
Calculation Methods
Calculating percent abundance involves several steps:
- Identify the isotopes of the element.
- Measure the mass of each isotope.
- Calculate the weighted average of the isotopes’ masses.
- Solve for the percent abundance using algebraic methods if necessary.
This process may require solving a system of equations if the percent abundances of multiple isotopes are unknown.
Applications
In scientific research, percent abundance is pivotal in fields like chemistry, physics, and geology. It aids in understanding atomic structure, nuclear reactions, and the formation of elements in the universe.
Relative Abundance
Concept Overview
Relative abundance measures the presence of species or isotopes in a given context, crucial for understanding ecosystem health and species distribution. Unlike percent abundance, it focuses on ratios, providing a scalable measure that can compare different systems or conditions.
Calculation Methods
To calculate relative abundance in ecological studies, follow a comparative approach:
- Count the number of individuals for each species.
- Total the counts for all species.
- Divide each species’ count by the total count to find its relative abundance.
This method offers a way to assess biodiversity and the impact of environmental changes on species populations.
Applications
Relative abundance is widely used in ecological and environmental studies to track changes in ecosystems, study the effects of pollution, and monitor conservation efforts. It helps scientists and policymakers understand which species are thriving, declining, or becoming invasive, guiding decisions on habitat protection and species conservation.

Key Differences
Definition and Usage
Percent and relative abundance, while similar in concept, serve distinct purposes. Percent abundance is primarily used in chemistry to denote the fraction of a particular isotope in a mixture of isotopes of an element, expressed as a percentage. It plays a critical role in determining the average atomic mass of elements. Relative abundance, however, is more often applied in ecology and environmental science, focusing on the proportion of a species within an ecosystem or a particular isotope among a group of isotopes, without the constraint of percentage values.
Distinct Aspects
The fundamental difference lies in their application and calculation. Percent abundance quantifies the presence of isotopes in a given sample and contributes to calculating an element’s atomic mass. Relative abundance, conversely, assesses the presence of species or isotopes relative to the total number, providing insights into biodiversity and isotopic distribution.
Calculation Contrast
- Percent abundance calculation involves algebraic methods and mass spectrometry data to determine the isotopic composition of an element.
- Relative abundance is calculated through a comparative approach, counting individual species or isotopes and comparing these numbers to the total observed.
Methodological differences
The method of calculation for each also underscores their unique roles. Percent abundance requires precise measurements and mathematical calculations to achieve accuracy, reflecting its importance in precise scientific analyses. Relative abundance, while also relying on quantitative data, often emphasizes comparative ratios that offer insights into ecological and environmental contexts.
Application Fields
- Percent abundance finds its utilization in fields requiring precise atomic mass calculations and isotopic compositions, such as chemistry and physics.
- Relative abundance is pivotal in ecology and environmental science, providing essential data for understanding ecosystem dynamics and species distribution.
Importance in Science
Isotopic Analysis
Isotopic analysis, a cornerstone of modern scientific research, relies heavily on understanding both percent and relative abundances. In geology, isotopic ratios are used to date rocks and minerals, offering a window into the Earth’s history. In archaeology, isotopic composition can reveal diet, migration patterns, and the origin of artifacts, underscoring the versatility and significance of isotopic studies.
Role in geological and archaeological studies
The role of isotopic analysis in these fields is profound. For instance, carbon dating, a method based on the relative abundance of carbon isotopes, allows scientists to determine the age of ancient organic materials. Similarly, isotopic signatures in mineral deposits can indicate the conditions under which they formed, providing valuable information about geological processes.
Environmental Monitoring
Tracking changes in ecosystems through relative abundance helps scientists understand the impact of human activity, climate change, and natural processes on biodiversity. This monitoring is crucial for developing strategies to protect endangered species and manage natural resources more sustainably.
Real-world Examples
Chemistry and Physics
- Isotope ratio analysis is a technique that uses the concept of percent abundance to measure the ratio of isotopes in a sample, providing insights into various physical processes and the history of the solar system. This analysis is fundamental in nuclear physics, geochemistry, and cosmochemistry.
Biology and Ecology
- In ecology, studies on species population utilize relative abundance to assess the health of ecosystems, the success of conservation efforts, and the impact of invasive species. For example, by tracking the relative abundance of native vs. invasive plant species, ecologists can understand the degree to which ecosystems are being altered and take steps to mitigate these changes.
Isotope Ratio Analysis
Isotope ratio analysis exemplifies the application of percent abundance in understanding natural processes and anthropogenic effects. For instance, the oxygen isotope ratio in ice cores can reveal past temperatures, contributing to our understanding of climate change. Similarly, strontium isotope ratios in human and animal remains provide data on ancient migration and diet.
Species Population Studies
Studying the relative abundance of species in various habitats allows for the assessment of biodiversity and ecosystem health. This data can inform conservation strategies, highlight species at risk, and indicate the overall balance of ecosystems. For example, shifts in the relative abundance of predator and prey species can signal changes in habitat quality and ecosystem dynamics, guiding conservation efforts.
Frequently Asked Questions
What is isotopic abundance?
Isotopic abundance refers to the proportion of an isotope present in a sample compared to the total amount of the element in all its isotopic forms. It provides essential information on the distribution of isotopes within a substance, crucial for fields like radiometric dating and environmental science.
How do you calculate percent abundance?
Percent abundance is calculated by dividing the amount of a specific isotope by the total amount of the element’s isotopes present in the sample, and then multiplying by 100 to express it as a percentage. This calculation gives a clear quantitative measure of an isotope’s presence relative to others.
Why is understanding relative abundance important?
Understanding relative abundance is vital for ecological and environmental studies as it helps quantify the distribution and diversity of species within ecosystems. It aids in assessing biodiversity, monitoring changes in species populations, and understanding the impacts of environmental stressors on ecological balance.
How do percent and relative abundance differ in application?
While percent abundance is primarily used in chemistry and physics to understand isotopic composition and its effects on material properties, relative abundance is more common in biology and ecology, focusing on species distribution and ecosystem health. The choice between these measures depends on the specific research objectives and the nature of the data being analyzed.
Conclusion
Grasping the difference between percent abundance and relative abundance is more than an academic exercise; it’s a cornerstone of accurate scientific analysis and interpretation. These concepts allow researchers to dissect the complex makeup of substances and ecosystems, offering insights that drive advancements in technology, environmental conservation, and our understanding of the natural world.
In conclusion, understanding these measures equips scientists and researchers with the tools necessary to make precise calculations, informed decisions, and contribute to their fields with rigorous, evidence-based science. As we continue to explore the natural world, the distinction between percent abundance and relative abundance will remain essential for unlocking the secrets of our universe’s material composition.
