Organic functional groups are cornerstone elements in the study and application of organic chemistry, significantly impacting molecular structure and reactivity. Among these groups, oxo and formyl play crucial roles, but their differences are often subtle yet significant. Recognized primarily for their participation in various chemical reactions, these groups influence a wide range of synthetic processes and biological functions.
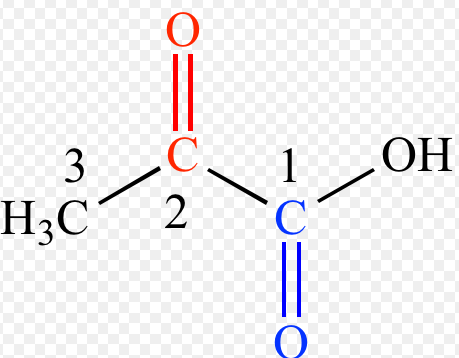
The oxo group is characterized by a carbonyl bonded to at least one hydrogen atom, commonly found in ketones and aldehydes. In contrast, the formyl group features a carbonyl bonded to a hydrogen atom, distinctively marking its presence in substances like formaldehyde and formic acid. This structural variance lays the foundation for their unique behaviors and applications in organic chemistry.
Both groups are essential in the synthesis of complex organic compounds, with the oxo group often participating in oxidation reactions while the formyl group is pivotal in formylation processes. Understanding their structural differences and chemical behaviors illuminates their diverse roles in industrial and biological contexts, reflecting their importance beyond mere structural components in organic molecules.
Basic Definitions
Oxo Group
Chemical Structure
The oxo group is a functional group in organic chemistry characterized by an oxygen atom double-bonded to a carbon atom, typically written as C=O. This group is central to many organic compounds, including ketones and aldehydes, where the carbon linked to the oxygen is also connected to other carbon atoms or hydrogen.
Common Occurrences
Oxo groups are ubiquitous in organic chemistry, found in:
- Ketones such as acetone, used widely as a solvent.
- Aldehydes like formaldehyde, essential in resin production and disinfectants. These compounds are integral to both biological processes and industrial applications, making the oxo group a cornerstone in synthetic organic chemistry.
Formyl Group
Chemical Structure
The formyl group features a carbonyl bond (C=O) attached directly to a hydrogen atom, symbolized as -CHO. This group forms the simplest aldehyde, formaldehyde, and is often a key intermediate in organic synthesis reactions.
Common Occurrences
Formyl groups are critical in:
- Formaldehyde, used in the production of resins and as a disinfectant.
- Formic acid, occurring naturally in ant venom and used industrially. These examples highlight the formyl group’s role in both nature and human-made chemical processes.
Chemical Properties
Oxo Group Properties
Reactivity
The oxo group is highly reactive due to the polar nature of the carbon-oxygen double bond, which can undergo various chemical reactions, including:
- Addition reactions, where molecules add across the double bond.
- Oxidation reactions, where the compound increases in oxygen content.
Chemical Behavior
In chemical environments, the oxo group’s behavior is marked by:
- Electrophilicity, where it acts as an electron-accepting group, making it reactive towards nucleophiles.
- Keto-enol tautomerism, a dynamic equilibrium important in many biochemical processes.
Formyl Group Properties
Reactivity
The formyl group’s reactivity is pronounced, particularly in:
- Nucleophilic attacks, where nucleophiles attack the positively polarized carbon.
- Reduction reactions, typically converting formyl groups into alcohols.
Chemical Behavior
Formyl groups exhibit specific behaviors such as:
- High electrophilicity, due to the partial positive charge on the carbonyl carbon.
- Formylation reactions, important in organic synthesis for adding formyl groups to other molecules.
Functional Differences
Oxo in Reactions
Role in Organic Synthesis
The oxo group plays a versatile role in organic synthesis, particularly in:
- Catalysis, where it facilitates various chemical transformations.
- Synthetic versatility, enabling the creation of a broad range of compounds.
Examples of Reactions
Notable reactions involving the oxo group include:
- Aldol Condensation, where two carbonyl compounds combine to form a larger molecule.
- Bayer-Villiger Oxidation, which transforms ketones into esters or lactones, demonstrating the oxo group’s critical role in expanding molecular complexity.
Formyl in Reactions
Role in Organic Synthesis
The formyl group is pivotal in organic synthesis due to its:
- Reactivity, making it an ideal candidate for initiating synthesis reactions.
- Utility in building blocks, acting as a starting point for synthesizing more complex molecules.
Examples of Reactions
Key reactions using the formyl group include:
- Vilsmeier-Haack reaction, where a formyl group is introduced to aromatic compounds.
- Reductive amination, where the formyl group is converted to an amine, illustrating its flexibility and importance in synthetic strategies.

Application Areas
Oxo Group Uses
Industrial Applications
The oxo group is fundamental in numerous industrial applications, contributing significantly to sectors such as plastics, solvents, and pharmaceuticals. Its ability to participate in various chemical reactions makes it invaluable in synthesizing:
- Plastics and polymers, where oxo groups facilitate the production of materials like polyvinyl chloride (PVC) and polyurethanes.
- Solvents, including acetone and butanone, used widely in manufacturing processes and chemical synthesis.
Biological Importance
In biological systems, oxo groups are crucial in:
- Metabolic pathways, where compounds like pyruvate and acetyl-coenzyme A play central roles in energy production.
- Signal transduction, as part of molecules that regulate biological processes.
Formyl Group Uses
Industrial Applications
The formyl group is key in the synthesis of:
- Pharmaceuticals, where formylation is used to modify the activity of bioactive compounds.
- Agricultural chemicals, including herbicides and pesticides, where formyl derivatives act as active ingredients.
Biological Importance
In nature, formyl groups are significant in:
- Metabolic reactions, particularly in the initiation of protein synthesis in prokaryotes.
- Cell signaling, where formyl peptides signal immune responses in inflammation and infection.
Comparative Analysis
Stability and Reactivity
Stability Differences
Comparing stability, oxo groups tend to be:
- More stable in varied environmental conditions than formyl groups.
- Less prone to spontaneous reactions, making them more manageable in controlled chemical processes.
Formyl groups, however, are:
- Generally more reactive, which, while useful in synthesis, can pose stability challenges.
Reactivity with Common Reagents
Both groups react with common reagents, yet differently:
- Oxo groups react predictably with nucleophiles and electrophiles, facilitating a range of synthetic transformations.
- Formyl groups are particularly susceptible to nucleophilic attack, due to the electron-deficient carbon, making them highly reactive in conditions favoring such reactions.
Synthesis Methods
Common Synthesis Pathways for Oxo
The synthesis of oxo groups in industrial settings often involves:
- Hydroformylation, combining alkenes, hydrogen, and carbon monoxide to produce aldehydes, which can be further processed into alcohols and acids.
- Oxidation reactions, where hydrocarbons are directly oxidized to form ketones or aldehydes, depending on the substrate and reaction conditions.
Common Synthesis Pathways for Formyl
Formyl groups are typically synthesized through:
- Vilsmeier-Haack reaction, a formylation method that converts aromatic compounds into aldehydes using chloroformates and DMF.
- Reformatsky reaction, involving zinc, aldehyde, and alpha-halo esters to form beta-hydroxy esters, incorporating formyl groups into more complex structures.
Impact in Organic Synthesis
Influence on the Synthesis of Complex Molecules
Both oxo and formyl groups significantly influence the synthesis of complex organic molecules. Their presence allows for:
- Functional diversity, where different functional groups can be introduced, altering physical, chemical, and biological properties.
- Structural complexity, enabling the construction of intricate molecular architectures necessary for advanced pharmaceuticals and high-performance materials.
Case Studies Highlighting Their Roles
Highlighting the importance of these groups, several case studies illustrate their impact:
- Synthesis of cholesterol-lowering drugs, where oxo groups are essential in forming the core structure.
- Development of new antibiotics, with formyl groups playing a crucial role in enhancing the activity and specificity of drug molecules against bacterial targets.
Frequently Asked Questions
What is an Oxo Group?
An oxo group in organic chemistry refers to a functional group containing at least one oxygen atom double-bonded to a carbon atom. This group is a defining feature in structures like ketones and aldehydes, influencing their chemical reactivity and physical properties.
What is a Formyl Group?
The formyl group consists of a carbonyl bond attached to a hydrogen atom, making it simpler than the oxo but crucial in organic synthesis. It’s a fundamental group in chemicals such as formaldehyde and plays a critical role in formylation reactions.
How do Oxo and Formyl Groups Differ?
While both oxo and formyl groups involve a carbonyl carbon, the oxo group is bonded to hydrogen or carbon, whereas the formyl group always attaches directly to hydrogen. This distinction affects their chemical behavior and reactivity in organic synthesis.
Where are Oxo and Formyl Groups Used?
Oxo groups are prevalent in the manufacture of plastics, solvents, and pharmaceuticals due to their reactivity. Formyl groups are essential in the production of perfumes, other chemicals, and as intermediates in pharmaceutical synthesis.
Conclusion
The exploration of oxo and formyl groups reveals their indispensable roles in the domain of organic chemistry. By distinguishing their structures and understanding their unique chemical behaviors, scientists and chemists can manipulate these groups to synthesize a variety of important organic compounds. The profound impact of these functional groups extends from laboratory research to real-world applications, underlining their significance in both academic study and industrial use.
These insights not only deepen our appreciation for organic chemistry but also enhance our capability to innovate within this field. As we continue to explore the vast potentials of these functional groups, their contributions to science and technology will likely expand, reflecting their foundational importance in modern chemistry.

